What To Know About The 2023 Eyedrop Recalls Understanding The Risks
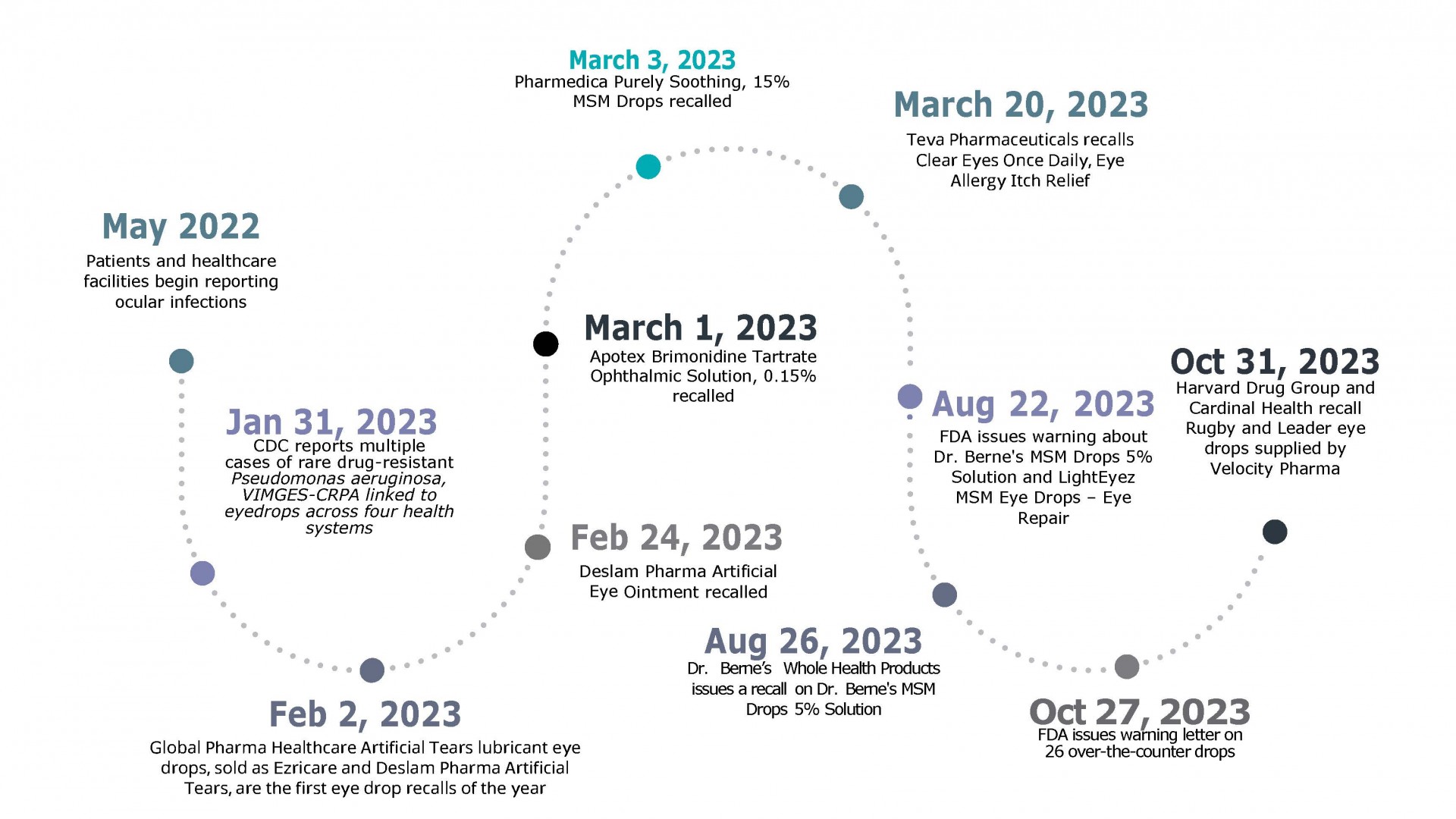
What To Know About The 2023 Eyedrop Recalls Understanding The Risks Global pharma healthcare drops were the first eye drops linked to pseudomonas aeruginosa, a bacterium commonly found in soil and water. on january 31, 2023, the cdc reported multiple cases of a rare, dangerous, and drug resistant p. aeruginosa, vim ges crpa. at the time, cdc investigators identified 55 cases across 12 states in the us. Choose single use drops: a 2022 research review showed that about 3% of multi use eye drop bottles become contaminated when used for less than one week compared with 24% of bottles used for more than one week. contamination occurs by touching the dropper cap or tip to a non sterile surface such as a countertop or the fingers, eyelashes, or.

What To Know About The 2023 Eyedrop Recalls Understanding The Risks Fda warns against using 26 eye drop products due to infection risk 00:33. the fda has expanded its list of eye drops recalled in 2023 because the products could be tainted with bacteria. The food and drug administration issued a warning in late october 2023 urging consumers to avoid purchasing and to immediately stop using 26 over the counter eye drop products because of risk of. The fda recently cautioned against using dozens of kinds of eyedrops — its third warning this year — leading to some wondering whether any drops are safe to use. eye doctors weigh in. Here's a full list of eye drops recalled in 2023: ezricare artificial tears lubricant eye drops, ndc 79503 0101 15 and upc 3 79503 10115 7. delsam pharma artificial tears lubricant eye drops, ndc.

Comments are closed.