What Is A Cancer Clinical Trial
Cancer Clinical Trials Cancer Care St Luke S Cancer Center You can donate your medical data and health samples >. see how donating your existing medical records and health samples can save lives. explains clinical trials, including what they are, why they are important, things to think about when deciding to take part, and questions to ask your doctor. Most cancer clinical trials are treatment studies that involve people who have cancer. these trials test new treatments or new ways of using existing treatments, including new: drugs. vaccines. approaches to surgery or radiation therapy. combinations of treatments. as researchers learn more about the genetic changes that lead to cancer, doctors.

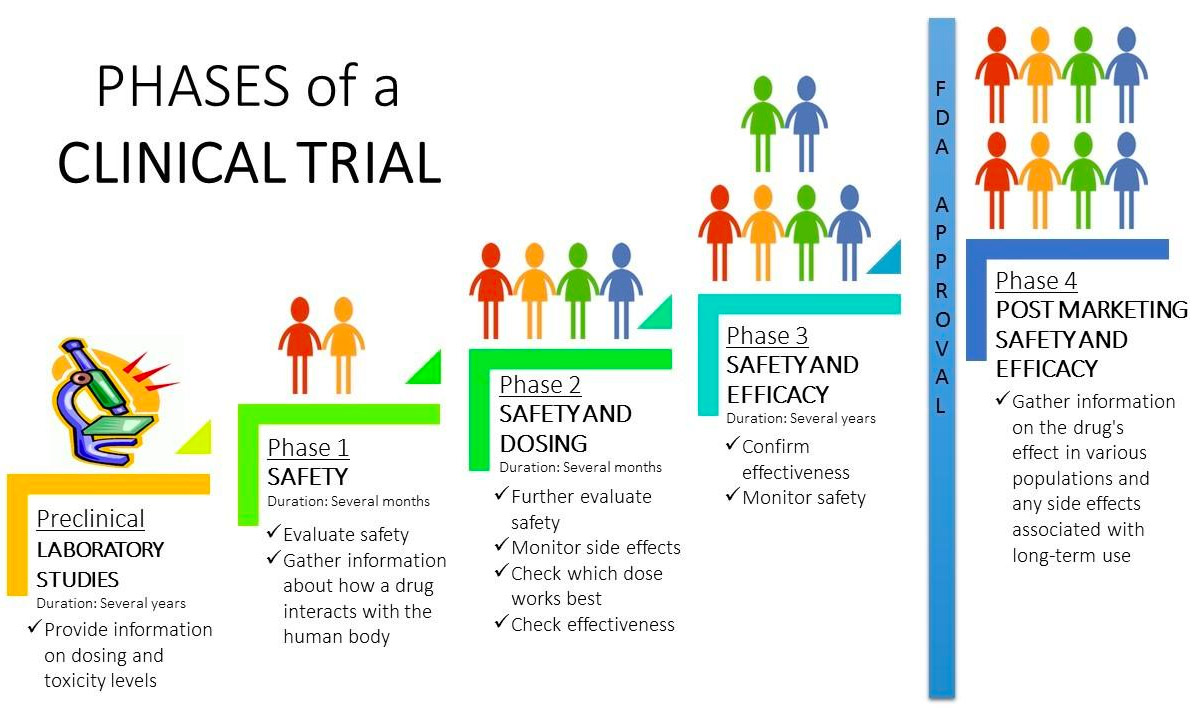
Cancer Treatment Clinical Trials Doctorvisit Overview. cancer clinical trials, also called research studies, test many types of treatments such as new drugs, new surgical techniques or radiation therapy, new combinations of treatments, or new methods. the goal of the research is to find better ways to treat cancer. cancer clinical trials include research at four different phases. Phase i trials usually include a small number of people (up to a few dozen). phase i trials most often include people with different types of cancer. these studies are usually done in major cancer centers. phase i trials carry the most potential risk. but phase i studies do help some patients. Clinical trials are studies of new drugs, procedures and other treatments in people. doctors use clinical trials to develop new treatments for serious diseases such as cancer. in this section you can learn about clinical trials in general, find tools to help you decide if a clinical trial may be right for you, and search for specific studies. The four phases of clinical trials. early clinical trial phases (phases 1 and 2) test for safety, such as what the side effects are and what a safe dose is. later phases (phase 3 and 4) compare the treatment to current standard treatments. in a phase 1 clinical trial, researchers figure out whether a new treatment is safe, what its side effects.

Comments are closed.