Water Distillation Process Diagram

Distillation Of A Product From A Reaction The Chemistry Blog Distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid form. it is exemplified at its simplest when steam from a kettle becomes deposited as drops of distilled water on a cold surface. distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic. Be sure to use a combination of both ice and water in the bath, as ice alone does not have as good contact with the flask as a slurry of ice water. figure 5.30: a) distillation with graduated cylinder receiving flask, b) distillation with receiving flask submerged in an ice bath, c) distillation using a claisen adapter, d) attachment of a.

Animated Water Distillation Process Diagram In Powerpoint Chemistry Pressure swing distillation takes advantage of the fact that boiling point (t,x) diagrams are two dimensional slices of a (t,x,p) diagram in which the pressure is the third variable. this means that the azeotropic composition depends on the pressure, so distillation at some pressure other than 1 atm may allow one to "jump" the azeotrope. The water distillation process: how does it work? a water distillation system is designed to purify water cheaply, quickly and effectively. to distill water, all you really need is a heat source and a condenser. since water has a lower boiling point than contaminants and minerals like salt, bacteria, heavy metals, calcium and phosphorus, when. A diagram of water distillation, the most simple thermal desalination process. here, a flame is applied to a beaker containing salt water; the water evaporates leaving the salts behind. Rounded lid for the pot. metal or glass bowl that floats inside the pot. ice cubes. fill the large pot partially full of the impure water. float the collection bowl on the water. the goal is to drip water from the inverted lid into this bowl, so make sure the bowl is large enough to catch the drips. place the pot lid upside down on the pot.

Water Distillation Process As Physics Method For Pure Water Extraction A diagram of water distillation, the most simple thermal desalination process. here, a flame is applied to a beaker containing salt water; the water evaporates leaving the salts behind. Rounded lid for the pot. metal or glass bowl that floats inside the pot. ice cubes. fill the large pot partially full of the impure water. float the collection bowl on the water. the goal is to drip water from the inverted lid into this bowl, so make sure the bowl is large enough to catch the drips. place the pot lid upside down on the pot. A water distiller is a special equipment designed to produce contaminant free water by transforming water into vapor before condensing into a liquid state. water distillers replicate the earth’s natural filtration process to produce water of unmatched purity. impurities, including germs, heavy metals, and arsenic, are removed during. Distillation is evaporation with subsequent condensation. the vapors formed are condensed by cooling, i.e., they are transformed from the gaseous state back to the liquid state. it is one of the oldest methods of separating liquid or molten substances. in the process of water purification, the source of water could be any type, e.g., be it.
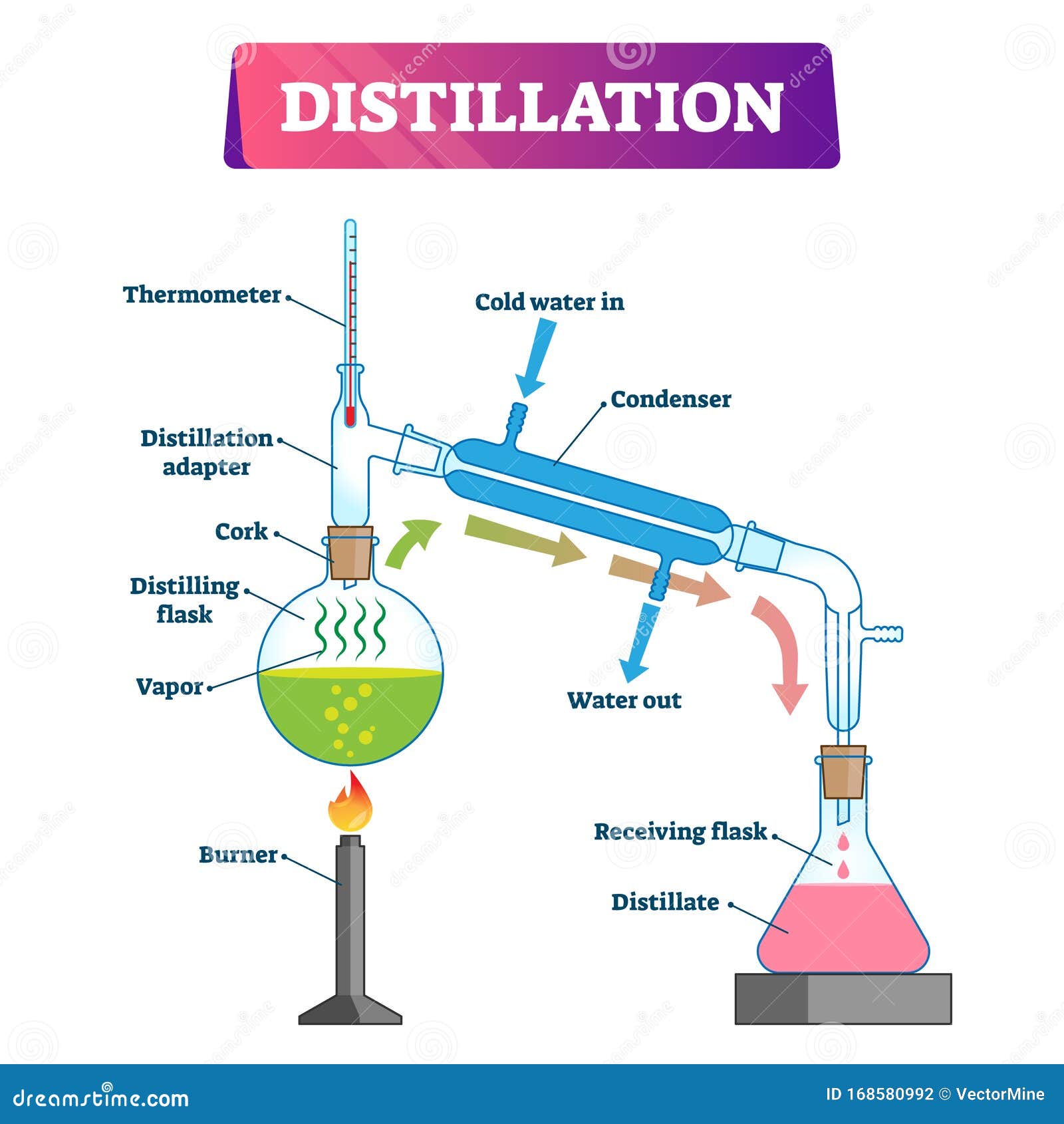
Water Distillation Process Diagram A water distiller is a special equipment designed to produce contaminant free water by transforming water into vapor before condensing into a liquid state. water distillers replicate the earth’s natural filtration process to produce water of unmatched purity. impurities, including germs, heavy metals, and arsenic, are removed during. Distillation is evaporation with subsequent condensation. the vapors formed are condensed by cooling, i.e., they are transformed from the gaseous state back to the liquid state. it is one of the oldest methods of separating liquid or molten substances. in the process of water purification, the source of water could be any type, e.g., be it.

Comments are closed.