Vaccine Basics Covid19
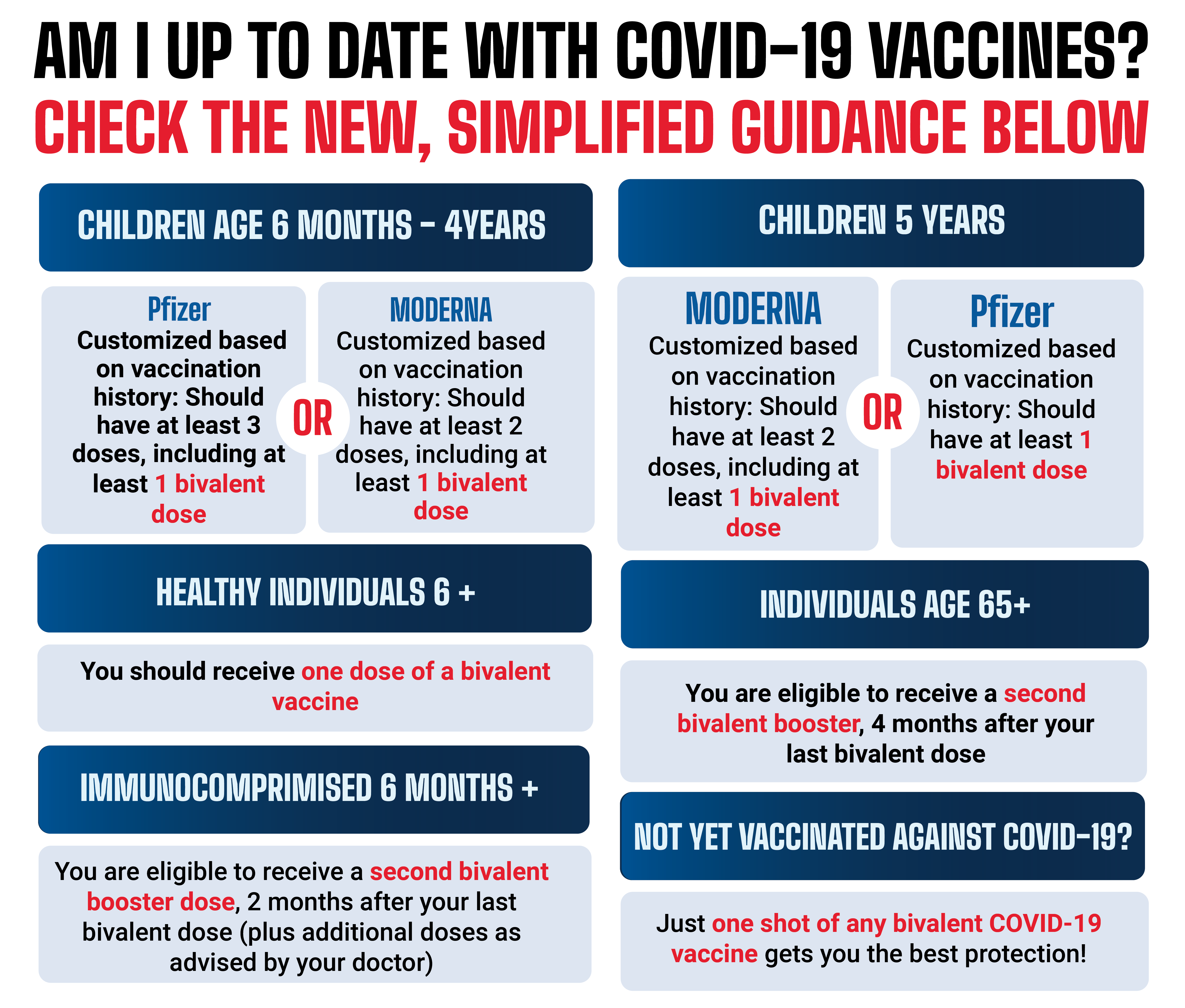
Vaccine Basics Covid19 There are different types of vaccines. all covid 19 vaccines prompt our bodies to recognize and help protect us from the virus that causes covid 19. currently, there are two types of covid 19 vaccines for use in the united states: mrna, and protein subunit vaccines. none of these vaccines can give you covid 19. vaccines do not use any live virus. Some people have no side effects from the covid 19 vaccine. for those who get them, most side effects go away in a few days. a covid 19 vaccine can cause mild side effects after the first or second dose. pain and swelling where people got the shot is a common side effect. that area also may look reddish on white skin.
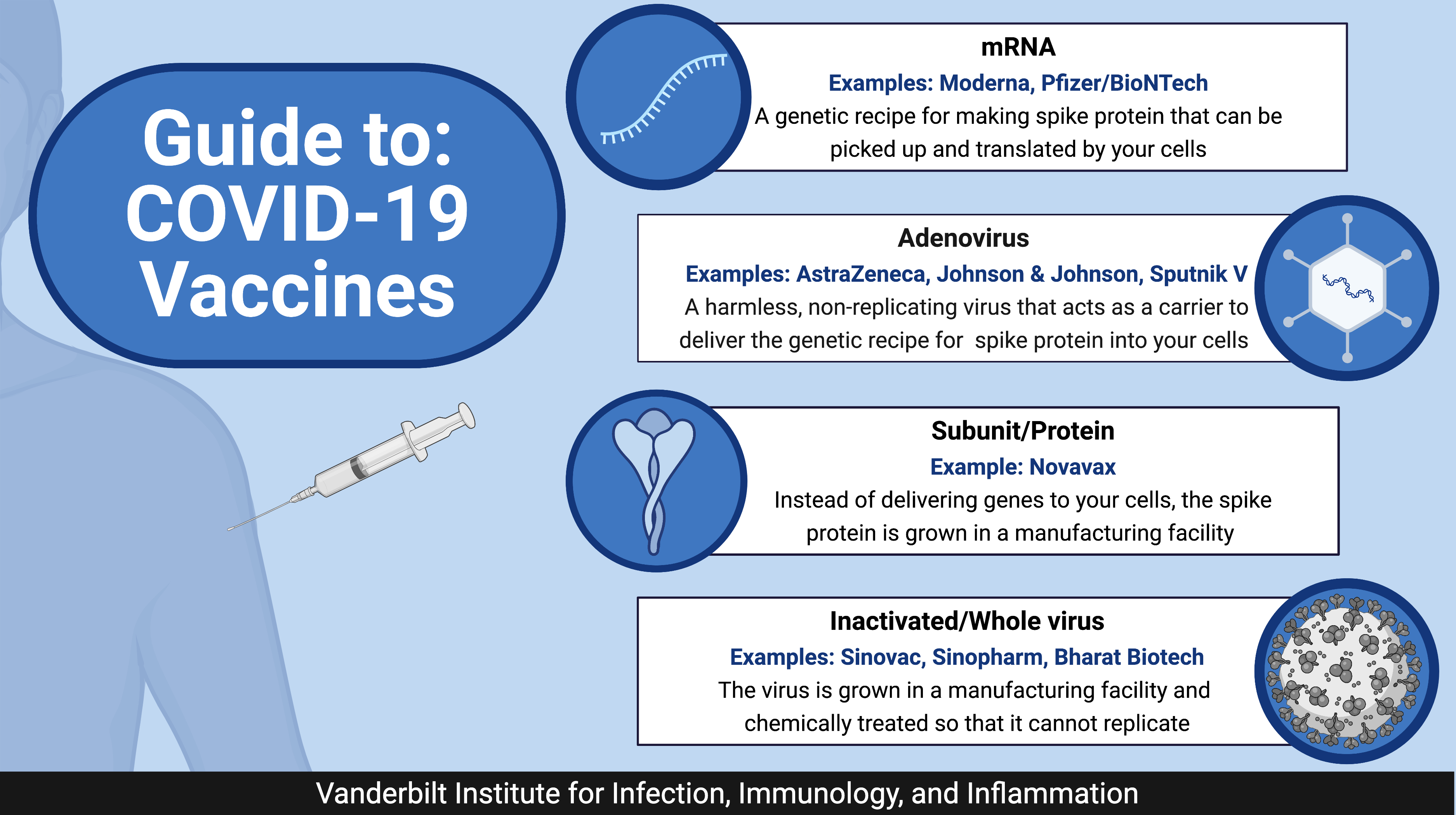
Guide To Covid 19 Vaccines Vanderbilt Institute For Infection This type of covid 19 vaccine contains harmless s proteins. once your immune system recognizes the s proteins, it creates antibodies and defensive white blood cells. if you later become infected with the covid 19 virus, the antibodies will fight the virus. the novavax covid 19 vaccine is a protein subunit vaccine. Because of the urgent need for a covid 19 vaccine, initial clinical trials of vaccine candidates were performed with the shortest possible duration between doses. therefore an interval of 21–28 days (3–4 weeks) between doses is recommended by who. depending on the vaccine, the interval may be extended for up to 42 days – or even up to 12. The mrna vaccines for covid 19 are made by the pfizer and moderna companies. it is available to people age 6 months and older. recombinant protein vaccine – this type of vaccine contains a version of a specific protein that is found in the virus. it is combined with another ingredient to help trigger the immune system. Currently approved covid 19 vaccines, including those based on the index virus and the bivalent vaccines, continue to protect against severe disease. for people at a high risk of getting severe covid 19, a dose of any available vaccine is more beneficial than delaying vaccination. many covid 19 manufacturers have developed or are in the process.
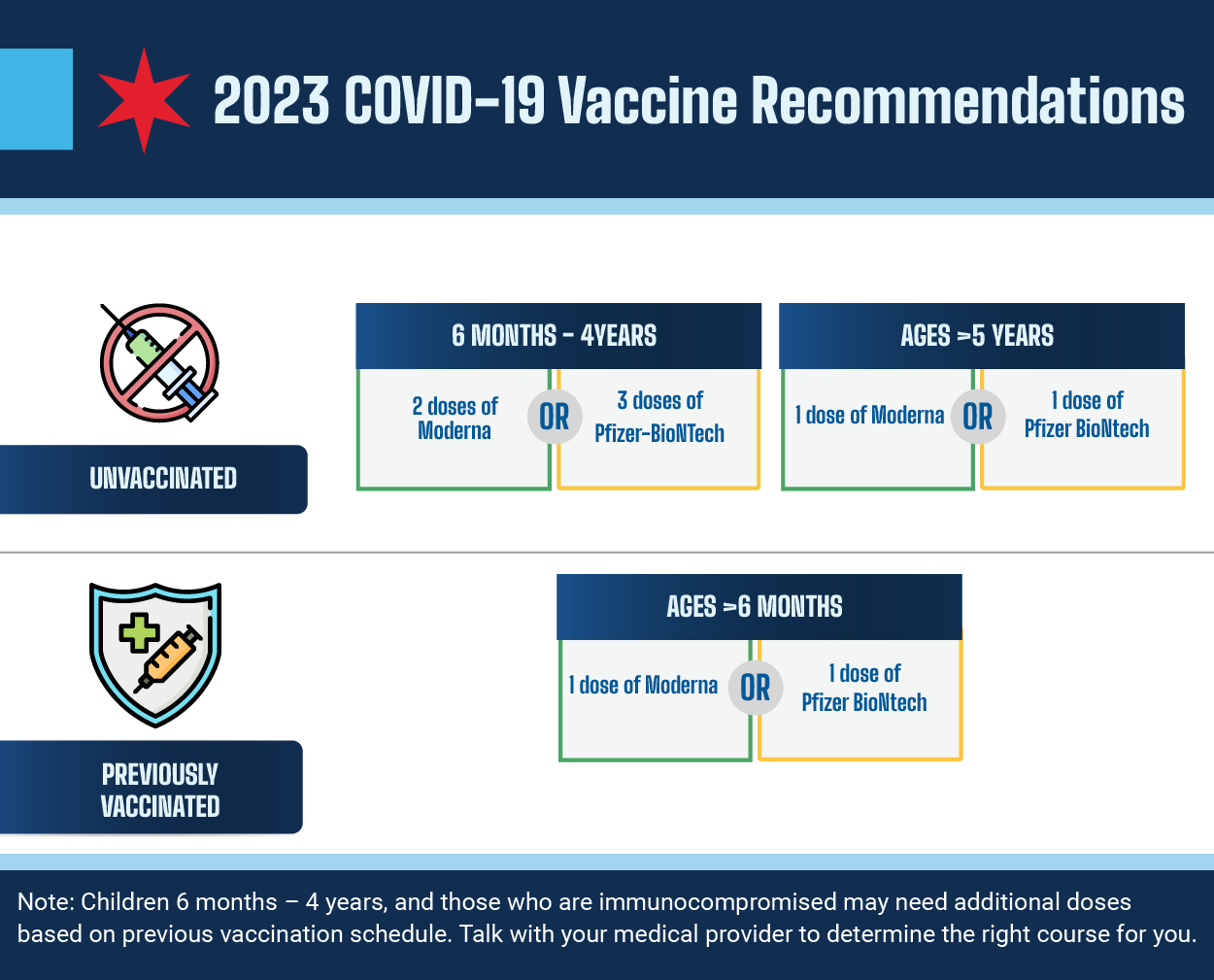
Vaccine Basics Covid19 The mrna vaccines for covid 19 are made by the pfizer and moderna companies. it is available to people age 6 months and older. recombinant protein vaccine – this type of vaccine contains a version of a specific protein that is found in the virus. it is combined with another ingredient to help trigger the immune system. Currently approved covid 19 vaccines, including those based on the index virus and the bivalent vaccines, continue to protect against severe disease. for people at a high risk of getting severe covid 19, a dose of any available vaccine is more beneficial than delaying vaccination. many covid 19 manufacturers have developed or are in the process. Yes, all who emergency use listed or prequalified covid 19 vaccines provide protection against severe disease and death from circulating covid 19 variants. any of the approved covid 19 vaccines can be used either for the initial series or revaccination. vaccination should not be delayed in anticipation of newer versions of the covid 19 vaccine. Fda approved and authorized the 2024 2025 mrna covid 19 vaccines on august 22, 2024. fda authorized novavax covid 19 vaccine, adjuvanted (2024 – 2025 formula) under emergency use authorization on august 30, 2024. learn how you can stay up to date with your covid 19 vaccine: staying up to date with covid 19 vaccines.
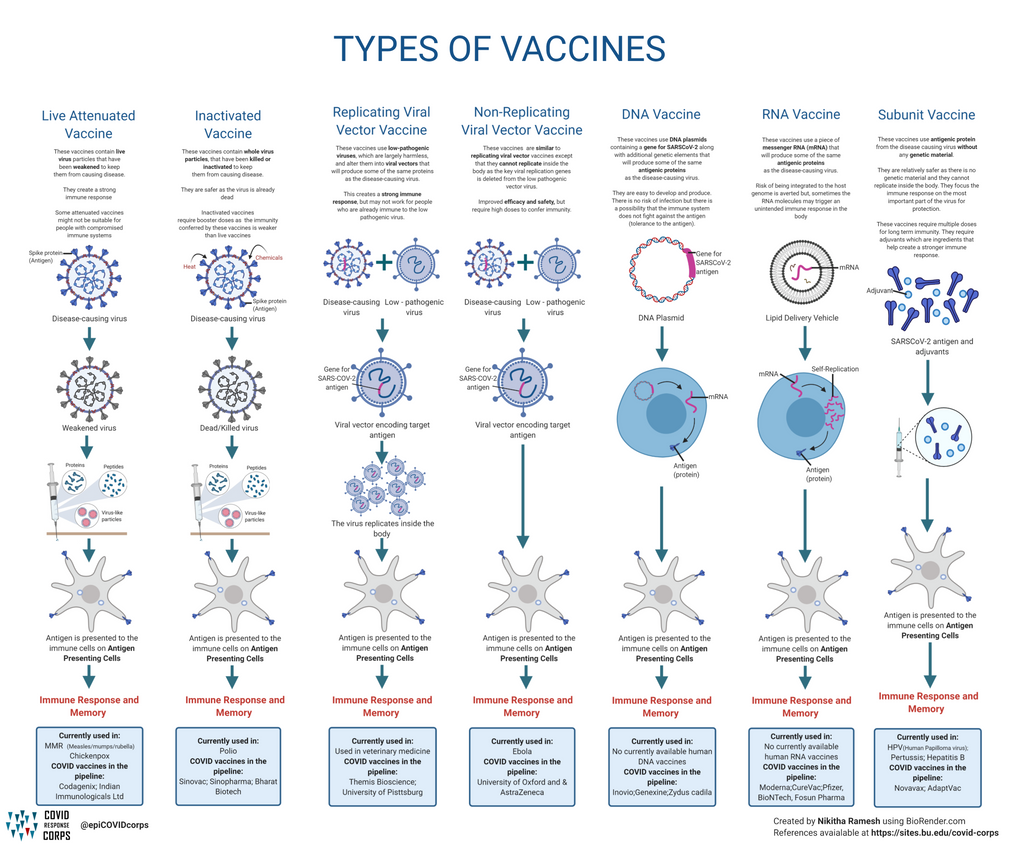
Types Of Vaccines Infographics Epidemiology Covid 19 Response Corps Yes, all who emergency use listed or prequalified covid 19 vaccines provide protection against severe disease and death from circulating covid 19 variants. any of the approved covid 19 vaccines can be used either for the initial series or revaccination. vaccination should not be delayed in anticipation of newer versions of the covid 19 vaccine. Fda approved and authorized the 2024 2025 mrna covid 19 vaccines on august 22, 2024. fda authorized novavax covid 19 vaccine, adjuvanted (2024 – 2025 formula) under emergency use authorization on august 30, 2024. learn how you can stay up to date with your covid 19 vaccine: staying up to date with covid 19 vaccines.
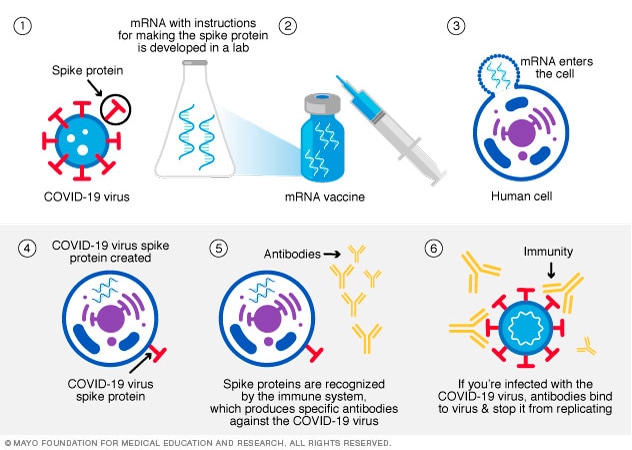
Diferentes Tipos De Vacunas Contra La Covid 19 Cгіmo Funcionan Mayo

Comments are closed.