Solved Solid Calcium Oxide Cao п їand Carbon Dioxide Gas Chegg
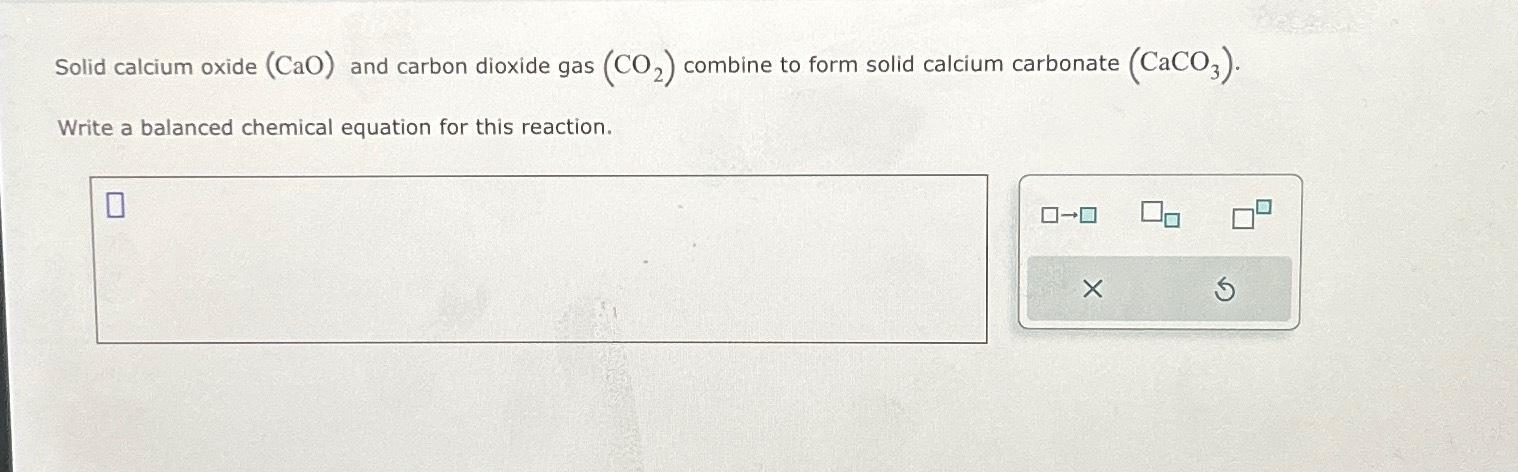
Solved Solid Calcium Oxide Cao п їand Carbon Dioxide Ga Solid calcium oxide (cao) and carbon dioxide gas (co2) combine to form solid calcium carbonate (caco3).write a balanced chemical equation for this reaction. your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. When solid calcium oxide is exposed to a stream of carbon dioxide gas, the following balanced chemical equation represents the reaction that takes place: cao (s) co2 (g) → caco3 (s) in this equation: cao represents solid calcium oxide. co2 represents carbon dioxide gas. caco3 represents solid calcium carbonate, the product formed from.

Solved Solid Calcium Oxide Cao And Carbon Dioxide Gasођ Word equation. calcium carbonate = calcium oxide carbon dioxide. caco3 = cao co2 is a decomposition reaction where one mole of calcium carbonate [caco 3] decomposes into one mole of calcium oxide [cao] and one mole of carbon dioxide [co 2]. Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. "approx." 80*g we need a stoichiometric reaction that represents the decomposition of calcium carbonate: caco 3(s) delta rarr cao(s) co 2(g)uarr and thus calcium oxide and calcium carbonate are present in equimolar amounts: "moles of calcium oxide" = (44.5*g) (56.08*g*mol^ 1)=0.794*mol. given the stoichiometry of the reaction, an equimolar quantity of calcium carbonate must have been. Caco3(s) = cao (s) co2 (g) when heated strongly, solid calcium carbonate decomposes to produce solid calcium oxide and carbon dioxide gas, as represented by the equation above. a 2.0 mol sample of caco3(s) is placed in a rigid 100. l reaction vessel from which all the air has been evacuated.

Solved A Mixture Of Solid Calcium Oxide Cao And Solid Cheggођ "approx." 80*g we need a stoichiometric reaction that represents the decomposition of calcium carbonate: caco 3(s) delta rarr cao(s) co 2(g)uarr and thus calcium oxide and calcium carbonate are present in equimolar amounts: "moles of calcium oxide" = (44.5*g) (56.08*g*mol^ 1)=0.794*mol. given the stoichiometry of the reaction, an equimolar quantity of calcium carbonate must have been. Caco3(s) = cao (s) co2 (g) when heated strongly, solid calcium carbonate decomposes to produce solid calcium oxide and carbon dioxide gas, as represented by the equation above. a 2.0 mol sample of caco3(s) is placed in a rigid 100. l reaction vessel from which all the air has been evacuated. Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate ($\ce{caco3}$; mineral calcite) in a lime kiln. this is accomplished by heating the material to above 825 °c (1,517 °f), a process called calcination or lime burning, to liberate a molecule of carbon dioxide. Limelight. quicklime (cao), compound of one atom of calcium and one atom of oxygen that is a white or grayish white solid produced in large quantities by roasting calcium carbonate so as to drive off carbon dioxide. at room temperature, cao will spontaneously absorb carbon dioxide from the atmosphere, reversing the reaction.

Comments are closed.