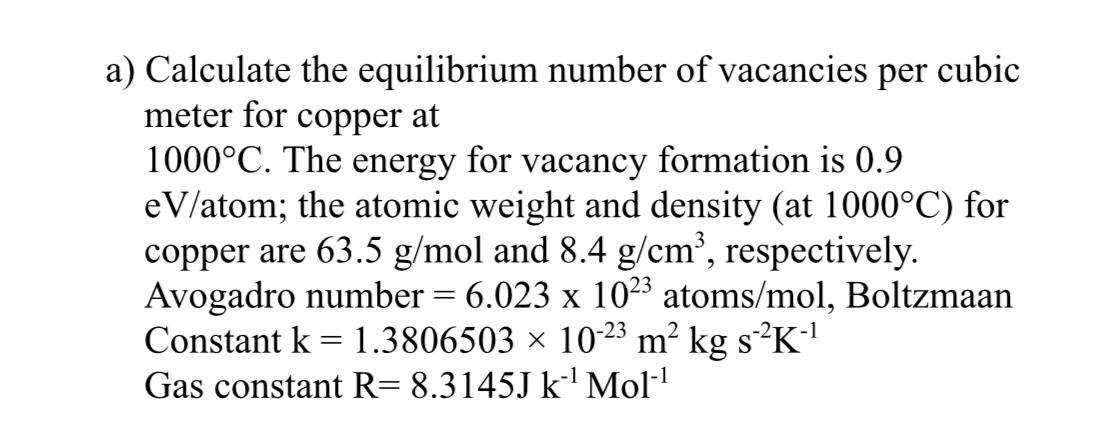
Solved Question 4 A Calculate The Equilibrium Number Of Vacancies

Solved Q4 Calculate The Equilibrium Number Of Vacancies Per Chegg Mechanical engineering questions and answers. 4. calculate the equilibrium number of vacancies per cm' for gold (au) @ 1000°c. given: energy for vacancy formation: 0.67 ev atom au atomic weight (a) = 196.96 g mol au density (p) = 19.3 g cm determine (n), the number of atomic sites per cm' for au. determine (n,), the number of vacancies @ 1000. Our expert help has broken down your problem into an easy to learn solution you can count on. question: q4. calculate the equilibrium number of vacancies per cubic meter for copper at 1000 °c. the energy for vacancy formation is 0.9 ev atom; the atomic weight and density (at 1000 °c) for copper are 63.5 g mol and 8.4 g cm', respectively.
Solved A Calculate The Equilibrium Number Of Vacancies Per Chegg Mae 20 winter 2011 assignment 3 solutions. 4.3 calculate the activation energy for vacancy formation in aluminum, given that the equilibrium number of vacancies at 500°c (773 k) is 7.57 × 1023 m 3. the atomic weight and density (at 500°c) for aluminum are, respectively, 26.98 g mol and 2.62 g cm3. Vacancy defect calculation #. the following is a calculation of the number of vacancy defects in a material. all crystalline and poly crystalline materials at temperatures above absolute zero contain vacancy defects. we can calculate the number of vacancies present in a material using the vacancy concentration equation below: n v n = e − q v r t. There are 2 steps to solve this one. experts have been vetted by chegg as specialists in this subject. calculate the equilibrium number of vacancies per cubic meter for silver (ag) at 600∘c. the energy for vacancy formation is 1.2ev atom. assume a density of 10.35 g cm3 and an atomic weight of 107.87 g mol for ag. The equilibrium number of vacancies can be calculated using the following equation: nv = nexp ( qv rt), where n is the total number of lattice sites, qv is the activation energy for vacancy formation, r is the gas constant, and t is the temperature in kelvin. 3.

Solved Calculate The Equilibrium Number Of Vacancies Per Chegg There are 2 steps to solve this one. experts have been vetted by chegg as specialists in this subject. calculate the equilibrium number of vacancies per cubic meter for silver (ag) at 600∘c. the energy for vacancy formation is 1.2ev atom. assume a density of 10.35 g cm3 and an atomic weight of 107.87 g mol for ag. The equilibrium number of vacancies can be calculated using the following equation: nv = nexp ( qv rt), where n is the total number of lattice sites, qv is the activation energy for vacancy formation, r is the gas constant, and t is the temperature in kelvin. 3. Assuming that the activation energy for vacancy formation in silver is 0.9 ev, and the density of silver is 10.5 g cm3, we can calculate the equilibrium number of vacancies at 800°c (1073 k) using the following values: nv n = exp( 0.9 ev (1.38 x 10^ 23 j k x 1073 k)) = 1.5 x 10^ 5 this means that for every 100,000 atoms in the silver lattice. The energy for vacancy formation is 0.9 ev atom; the atomic weight for cu is 63.5 g mol (assume it’s constant); the density is 8.4 g cm3 (assume it’s constant calculate the equilibrium number of vacancies per cubic meter for cu at 0k, rt (300k), 1000dc and 1350 k, six degrees below the melting point.

Solved Calculate The Equilibrium Number Of Vacancies Per Chegg Assuming that the activation energy for vacancy formation in silver is 0.9 ev, and the density of silver is 10.5 g cm3, we can calculate the equilibrium number of vacancies at 800°c (1073 k) using the following values: nv n = exp( 0.9 ev (1.38 x 10^ 23 j k x 1073 k)) = 1.5 x 10^ 5 this means that for every 100,000 atoms in the silver lattice. The energy for vacancy formation is 0.9 ev atom; the atomic weight for cu is 63.5 g mol (assume it’s constant); the density is 8.4 g cm3 (assume it’s constant calculate the equilibrium number of vacancies per cubic meter for cu at 0k, rt (300k), 1000dc and 1350 k, six degrees below the melting point.

Comments are closed.