Solved Problem 1 Calculate The Activation Energy For Chegg
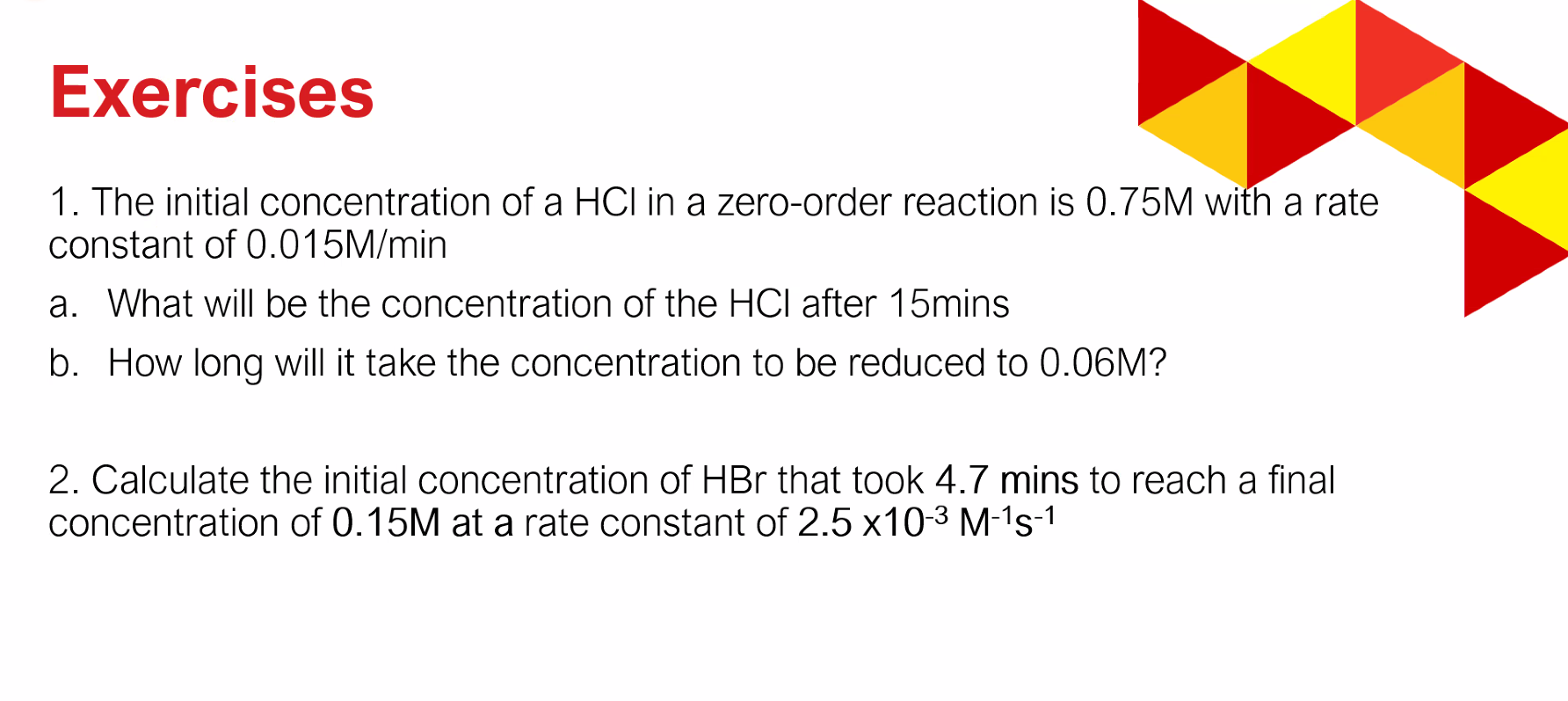
Solved Sample Problem 1 Calculate The Activation Energy Chegg Problem #1 calculate the activation energy for vacancy formation in aluminum, given that the equilibrium number of vacancies at 500°c (773 k) is 7.57 x 1023 m². the atomic weight and density (at 500°c) for aluminum are, respectively, 26.98 g mol and 2.62 g cm?. Sample problem 1. calculate the activation energy from the given data ln k = − r e a t 1 ln a set the slope equal to : r − e a 1. the initial concentration of a hcl in a zero order reaction is 0.75 m with a rate constant of 0.015 m min a. what will be the concentration of the hcl after 15 mins b.

Solved 1 Calculate The Activation Energy For The Elementary Cheggођ Activation energy formula solution. the activation energy can be determined using the equation: ln (k 2 k 1) = e a r x (1 t 1 1 t 2) where. e a = the activation energy of the reaction in j mol. r = the ideal gas constant = 8.3145 j k·mol. t 1 and t 2 = absolute temperatures (in kelvin) k 1 and k 2 = the reaction rate constants at t 1 and t 2. From the plot of \(\ln f\) versus \(1 t\), calculate the slope of the line (−e a r) and then solve for the activation energy. express equation \ref{14.5.4} in terms of k 1 and t 1 and then in terms of k 2 and t 2. subtract the two equations; rearrange the result to describe k 2 k 1 in terms of t 2 and t 1. To calculate the activation energy from a graph: draw ln k (reaction rate) against 1 t (inverse of temperature in kelvin). find the slope of the line m knowing that m = eₐ r, where eₐ is the activation energy, and r is the ideal gas constant. or: make a plot of the energy of the reaction versus the reaction progress. The activation energy can also be found algebraically by substituting two rate constants (k 1, k 2) and the two corresponding reaction temperatures (t 1, t 2) into the arrhenius equation (2). substracting equation (4) from equation (3) results in rerrangement of equation (5) and solving for e a yields . let's try a problem:.
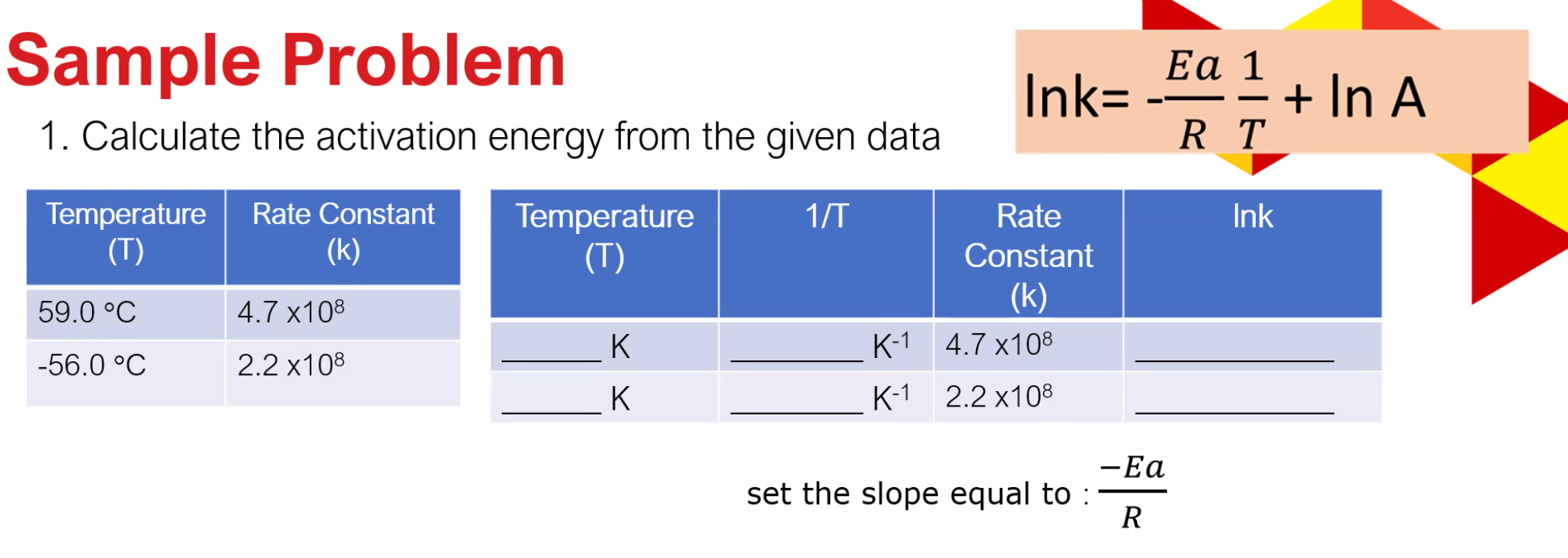
Solved Sample Problem 1 Calculate The Activation Energy Chegg To calculate the activation energy from a graph: draw ln k (reaction rate) against 1 t (inverse of temperature in kelvin). find the slope of the line m knowing that m = eₐ r, where eₐ is the activation energy, and r is the ideal gas constant. or: make a plot of the energy of the reaction versus the reaction progress. The activation energy can also be found algebraically by substituting two rate constants (k 1, k 2) and the two corresponding reaction temperatures (t 1, t 2) into the arrhenius equation (2). substracting equation (4) from equation (3) results in rerrangement of equation (5) and solving for e a yields . let's try a problem:. Figure 6.2.3.3.1: lowering the activation energy of a reaction by a catalyst. this graph compares potential energy diagrams for a single step reaction in the presence and absence of a catalyst. the only effect of the catalyst is to lower the activation energy of the reaction. the catalyst does not affect the energy of the reactants or products. Problem\#1: calculate the activation energy for vacancy formation in aluminum, given that the equilibrium number of vacancies at 50 0 ∘ c (773 k) is 7.57 × 1 0 23 m − 3. the atomic weight and density (at 50 0 ∘ c) for aluminum are, respectively, 26.98 g mol and 2.62 g cm 3.
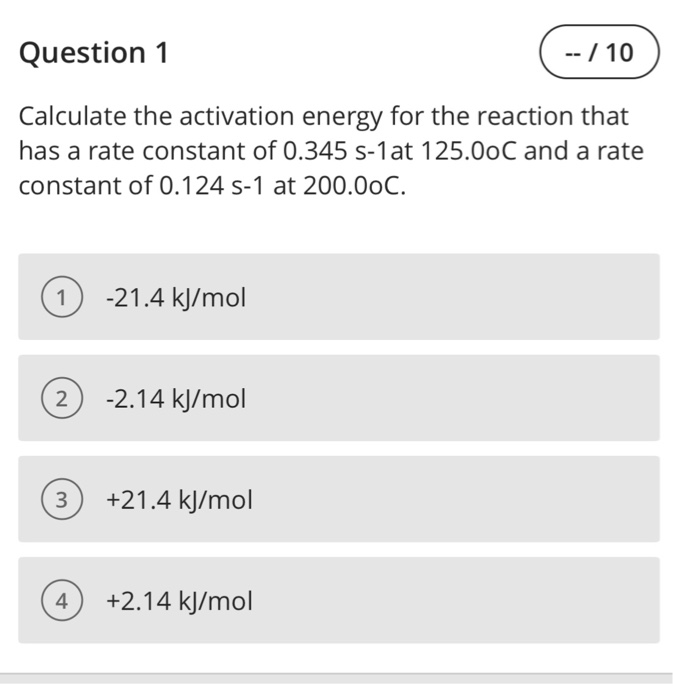
Solved Question 1 Calculate The Activation Energy For The Chegg Figure 6.2.3.3.1: lowering the activation energy of a reaction by a catalyst. this graph compares potential energy diagrams for a single step reaction in the presence and absence of a catalyst. the only effect of the catalyst is to lower the activation energy of the reaction. the catalyst does not affect the energy of the reactants or products. Problem\#1: calculate the activation energy for vacancy formation in aluminum, given that the equilibrium number of vacancies at 50 0 ∘ c (773 k) is 7.57 × 1 0 23 m − 3. the atomic weight and density (at 50 0 ∘ c) for aluminum are, respectively, 26.98 g mol and 2.62 g cm 3.

Comments are closed.