Report Sheet Experiment 1 Basic Laboratory Techniques Chegg
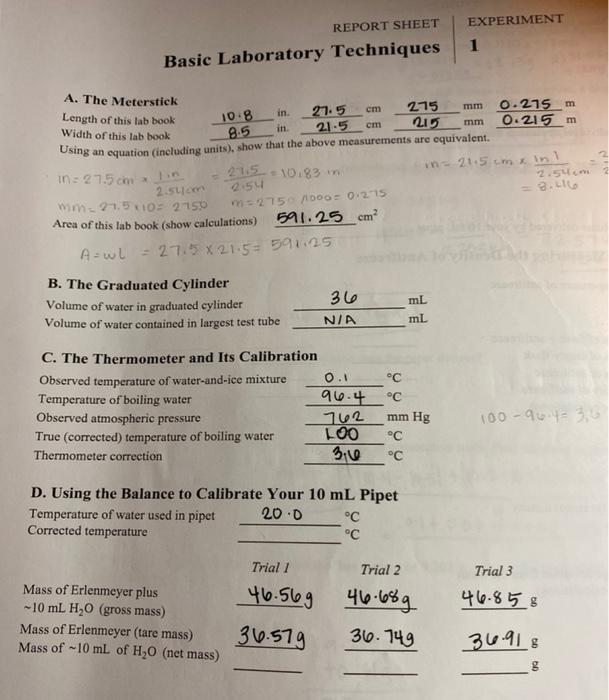
Report Sheet Experiment 1 Basic Laboratory Techniques Chegg Answer to report sheet experiment 1 basic laboratory techniques. report sheet experiment 1 basic laboratory techniques date w sec name desk no data and calculations a laboratory check in instructor approval b. laboratory safety instructor approval laboratory safety. Question: report sheet experiment basic laboratory techniques 1 a. the meterstick length of this lab book width of this lab book using an equation (including units), show that the above measurements are equivalent in. in. cm cm area of this lab book (show calculations) show transcribed image text. there are 2 steps to solve this one.

Solved Report Sheet Project 1 Laboratory Tools Experiment Chegg Chemistry questions and answers. report sheet experiment 1 basic laboratory techniques cm מות a. the meterstick length of this lab book width of this lab book 10:8 cm mm in. 21.5 275 0.275 m 8.5 in. 21 5 215 0.215 m using an equation (including units), show that the above measurements are equivalent. in = 27.5 cm in 21:5 =10.83 in 21.5cm. Study with quizlet and memorize flashcards containing terms like experiment 1: #3 measure the length of each of the following objects (cd or dvd, key, spoon, fork) with the ruler in centimeters (cm) to the correct level of precision and record in data table 1., #4 measure the temperature of the hot tap water with the thermometer in degrees celsius (°c) to the correct precision of the. D= m v. platinum = 21.43 10= 2.143. diamond = 3.51 10 = 0.351 ( diamond has most value) 8. expiremental procedure, part c.3. the mass of a beaker is 5.333g. after 5.00ml of a concentrated hydrochloric acid solution is pipetted into the beaker, the combined mass of the beaker and the hydrochloric acid sample is 11.229g. Explain. the solid is not fully submerged so it will make the reported density higher. since some of the solid is out of the water it will make the volume of the water displaced smaller. so, we would be dividing the mass by a smaller number equaling a smaller density. suppose that after delivery several drops of water cling to the inner wall of.

Chem 181 Experiment 1 Basic Laboratory Operations Chegg D= m v. platinum = 21.43 10= 2.143. diamond = 3.51 10 = 0.351 ( diamond has most value) 8. expiremental procedure, part c.3. the mass of a beaker is 5.333g. after 5.00ml of a concentrated hydrochloric acid solution is pipetted into the beaker, the combined mass of the beaker and the hydrochloric acid sample is 11.229g. Explain. the solid is not fully submerged so it will make the reported density higher. since some of the solid is out of the water it will make the volume of the water displaced smaller. so, we would be dividing the mass by a smaller number equaling a smaller density. suppose that after delivery several drops of water cling to the inner wall of. Laboratory report experiment 1. laboratory techniques and data analysis. chem 134 laboratory section # name: date: 002. 4 29 laboratory techniques and data analysis. introduction. in order for any experimental data to be of value, the data must have been obtained using reliable methods. This document describes experiment 1 on basic laboratory techniques, including calibrating a volumetric flask, pipette, and burette to determine their actual volumes. the results found minor differences between the actual and theoretical volumes, likely due to parallax errors when taking readings. proper technique like keeping the eye level when measuring is important to reduce errors and get.

Comments are closed.