Reaction Of Magnesium With Oxygen Gas 2mg O2 в 2mgo Reaction Of

Reaction Of Magnesium With Oxygen Gas 2mg O2 в 2mgo Rea Step 4: substitute coefficients and verify result. count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges ions) are balanced. since there is an equal number of each element in the reactants and products of 2mg o2 = (2mgo), the equation is balanced. The stoichiometry calculator above shows the mole ratios coefficients of the balanced equation, (2mg) o2 = (2mgo). enter the amount of any of the substances to determine the ideal amounts to maximize the theoretical yield of the reaction. to find the limiting and excess reagents when a non ideal amount of each substance is used, you can use.

Reaction Of Magnesium With Oxygen Mg O2 Youtube Magnesium reacts with oxygen as shown in the equation below: #2mg o 2 >2mgo# calculate the percentage yield of the reaction, given that burning 2.32g of magnesium produced 2.39g of magnesium oxide. 2 mg (s) o "2" (g) > 2 mgo first, we need to identify the type of bond and reaction. this is an ionic bond so atoms are giving and accepting electrons. this is a synthesis reaction. magnesium has a charge of 2 , while oxygen has a charge of 2 . the burning of magnesium metal reacts with oxygen found in the air (oxygen gas) to form magnesium oxide. 2 mg (s) o "2" (g) > 2 mgo oxygen is a. The stoichiometry calculator above shows the mole ratios coefficients of the balanced equation, 2mg o2 = 2mgo. enter the amount of any of the substances to determine the ideal amounts to maximize the theoretical yield of the reaction. to find the limiting and excess reagents when a non ideal amount of each substance is used, you can use the. After it burns, it forms a white powder of the magnesium oxide. magnesium gives up two electrons to oxygen atoms to form this powdery product. this is an exothermic reaction. an exothermic reaction is a term that describes a chemical reaction in which there is a net release of energy (heat). 2mg(s) o2 (g) → 2mgo(s) energy (1) (1) 2 m g (s.
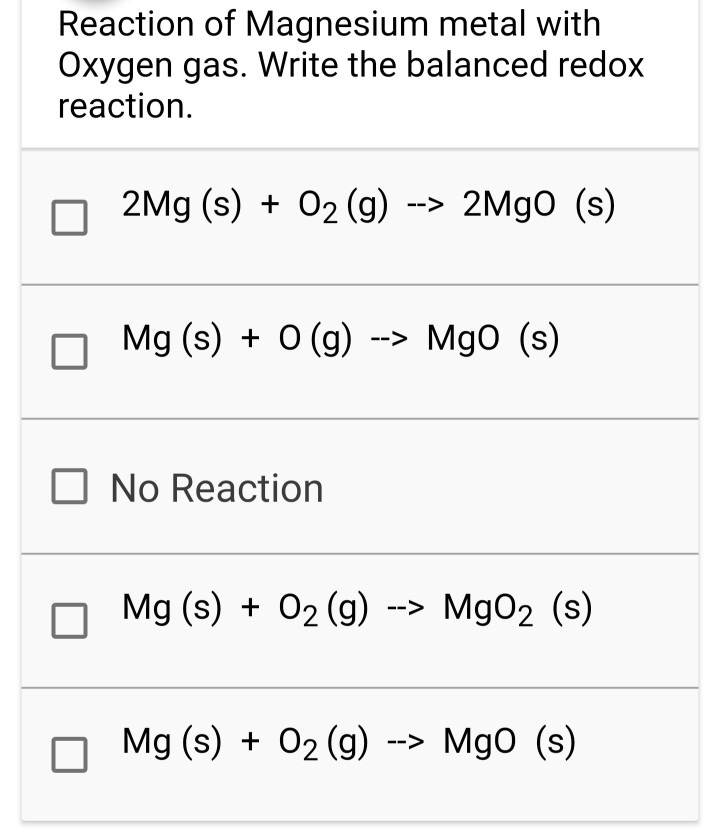
Solved Reaction Of Magnesium Metal With Oxygen Gas Write Chegg The stoichiometry calculator above shows the mole ratios coefficients of the balanced equation, 2mg o2 = 2mgo. enter the amount of any of the substances to determine the ideal amounts to maximize the theoretical yield of the reaction. to find the limiting and excess reagents when a non ideal amount of each substance is used, you can use the. After it burns, it forms a white powder of the magnesium oxide. magnesium gives up two electrons to oxygen atoms to form this powdery product. this is an exothermic reaction. an exothermic reaction is a term that describes a chemical reaction in which there is a net release of energy (heat). 2mg(s) o2 (g) → 2mgo(s) energy (1) (1) 2 m g (s. And when 7.5*g metal are combusted, this should result in a heat output of (7.5*g) (24.3*g*mol^ 1)xx 602*kj*mol^ 1= 185.8*kj finally, you have proposed that a given mass of mgo is decomposed to magnesium metal, and oxygen gas. clearly, the energy here must be supplied to the system for decomposition. Consider the balanced reaction of magnesium and oxygen. 2mg o2 2mgo what mass, in grams, of mgo can be produced from 1.79 g of mg and 2.68 g of o2? your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on.

Solved Consider The Balanced Reaction Of Magnesium And 2mg 50 Off And when 7.5*g metal are combusted, this should result in a heat output of (7.5*g) (24.3*g*mol^ 1)xx 602*kj*mol^ 1= 185.8*kj finally, you have proposed that a given mass of mgo is decomposed to magnesium metal, and oxygen gas. clearly, the energy here must be supplied to the system for decomposition. Consider the balanced reaction of magnesium and oxygen. 2mg o2 2mgo what mass, in grams, of mgo can be produced from 1.79 g of mg and 2.68 g of o2? your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on.

Reaction Of Magnesium With Oxygen Gas Burning Magnesium In Air Youtube

Comments are closed.