Ppt Chapter 19 Entropy And Free Energy Powerpoint Presentation о

Ppt Chapter 19 Entropy And Free Energy Powerpoint Prese Vibrational energy and entropy general chemistry: chapter 19. 19 4 criteria for spontaneous change:the second law of thermodynamics. Δstotal = Δsuniverse = Δssystem Δssurroundings the second law of thermodynamics: Δsuniverse = Δssystem Δssurroundings > 0 all spontaneous processes produce an increase in the entropy of the universe. Entropy • entropy (from greek, meaning • “in transformation”) • is a thermodynamic property • that relates • the distribution of the total energy of the system • to the available energy levels of the particles. entropy • a general way to envision entropy is • “differing ways to move” • consider mountain climbers on a.
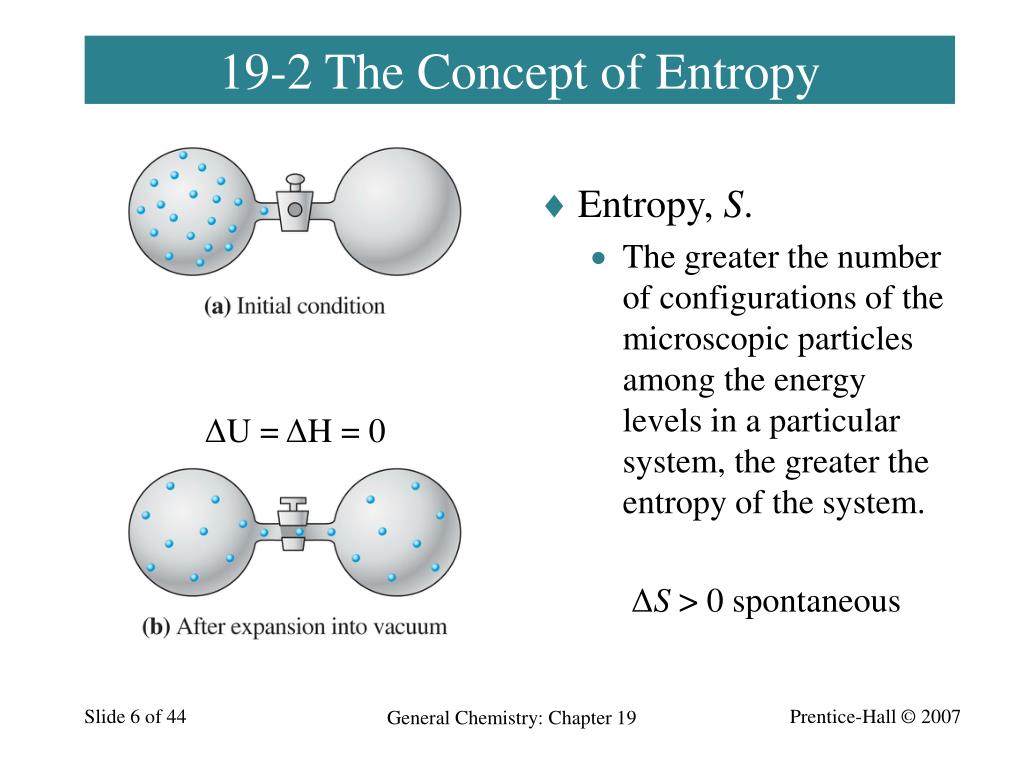
Ppt Chapter 19 Spontaneous Change Entropy And Free Energy Entropy and free energy. entropy and free energy. 19.3. after reading section 19.3, you should know:. what entropy and free energy are how to determine whether the entrophy is increasing or decreasing based on a chemical equation how to determine whether a reaction will be spontaneous or not. 421 views • 12 slides. Download ppt "chapter 17 spontaneity, entropy, and free energy". table of contents (17.1) spontaneous processes and entropy (17.2) entropy and the second law of thermodynamics (17.3) the effect of temperature on spontaneity (17.4) free energy (17.5) entropy changes in chemical reactions (17.6) free energy and chemical reactions (17.7) the. Entropy is a critical component of the laws of thermodynamics: 1st law: total energy of the universe is constant. 2nd law: the total entropy of the universe is always increasing. 3rd law: the entropy of a pure, perfectly formed crystalline substance at absolute zero is zero. so, since the minimum value for s is zero, s for any material is. 2) enthalpy is a measure of the total energy of a system and depends on internal energy and pressure volume work. entropy quantifies the disorder or randomness in a system. 3) free energy determines whether chemical reactions occur spontaneously and accounts for both enthalpy and entropy changes. reactions are spontaneous when the change in.

Ppt Chapter 19 Spontaneous Change Entropy And Free Energy 3b5 Entropy is a critical component of the laws of thermodynamics: 1st law: total energy of the universe is constant. 2nd law: the total entropy of the universe is always increasing. 3rd law: the entropy of a pure, perfectly formed crystalline substance at absolute zero is zero. so, since the minimum value for s is zero, s for any material is. 2) enthalpy is a measure of the total energy of a system and depends on internal energy and pressure volume work. entropy quantifies the disorder or randomness in a system. 3) free energy determines whether chemical reactions occur spontaneously and accounts for both enthalpy and entropy changes. reactions are spontaneous when the change in. To print or download this file, click the link below: chapter 17 spontaneity, entropy, and free energy.ppt — application vnd.ms powerpoint, 4.64 mb (4864512 bytes). Chemistry, the central science, 10th edition theodore l. brown; h. eugene lemay, jr.; and bruce e. bursten chapter 19 chemical thermodynamics john d. bookstaver – a free powerpoint ppt presentation (displayed as an html5 slide show) on powershow id: 6eca63 mgnmy.
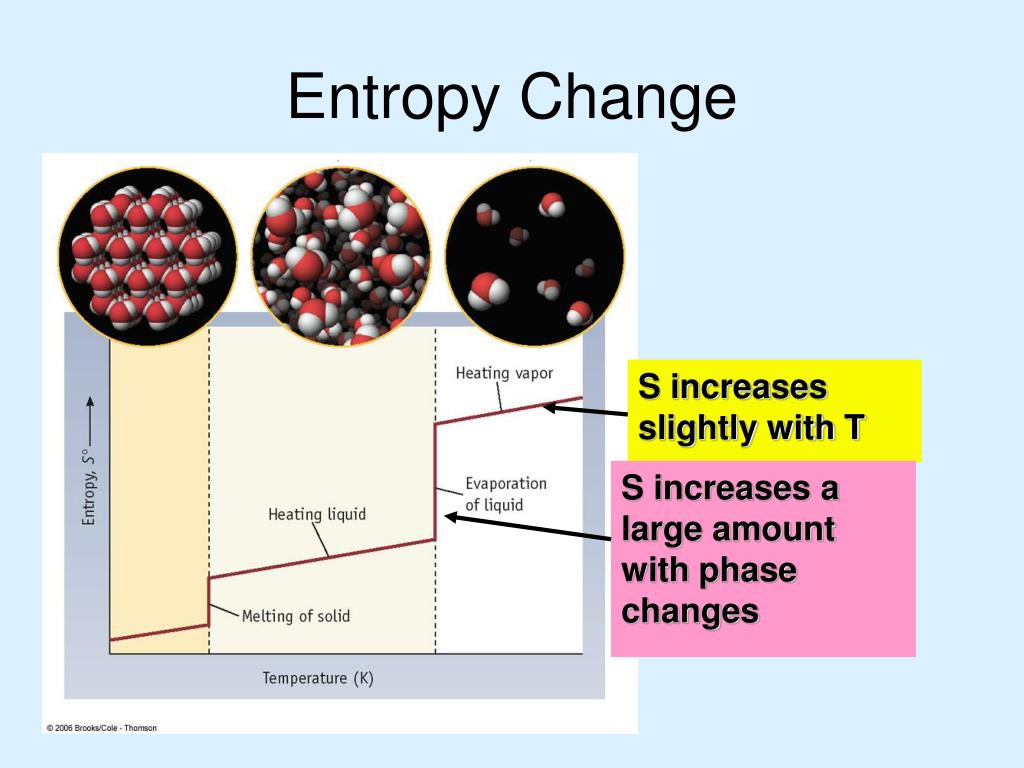
Ppt Chapter 19 Entropy And Free Energy Powerpoint Prese To print or download this file, click the link below: chapter 17 spontaneity, entropy, and free energy.ppt — application vnd.ms powerpoint, 4.64 mb (4864512 bytes). Chemistry, the central science, 10th edition theodore l. brown; h. eugene lemay, jr.; and bruce e. bursten chapter 19 chemical thermodynamics john d. bookstaver – a free powerpoint ppt presentation (displayed as an html5 slide show) on powershow id: 6eca63 mgnmy.

Ppt Chapter 19 вђ Principles Of Reactivity Entropy And Free Energyо

Comments are closed.