Possible Electron Transitions Giving Rise To Characteristic X Rays
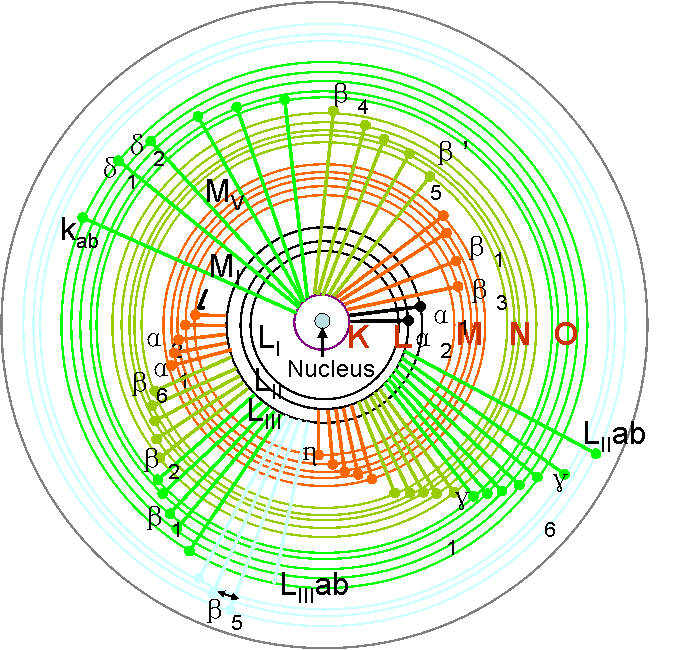
Possible Electron Transitions Giving Rise To Characteristic X Rays An outer shell electron will "drop down" to fill the void created in the inner shell and there is a certain probability that an x ray characteristic of that atom will be emitted. this process is called x ray fluorescence (xrf) and is shown in figure 3 (4). the characteristic x rays are called "k lines" if they result from an electron filling. These internal electron transitions give rise to the emission of "characteristic" x radiation which, because of its short wavelength, has extremely high "penetrating" power. since an electron beam is used to generate \(x\) rays, the \(x\) ray tube has to be evacuated: to dissipate the energy flux arriving at the target, the anode support (onto.
Possible Electron Transitions Giving Rise To Characteristic X Rays The different electron states which exist in an atom are usually described by atomic orbital notation, as is used in chemistry and general physics. however, x ray science has special terminology to describe the transition of electrons from upper to lower energy levels: traditional siegbahn notation, or alternatively, simplified x ray notation. Characteristic x rays are emitted from heavy elements when their electrons make transitions between the lower atomic energy levels. the characteristic x ray emission which is shown as two sharp peaks in the illustration at left occur when vacancies are produced in the n=1 or k shell of the atom and electrons drop down from above to fill the gap. The bohr model of this process is shown in figure 8.6.1 8.6. 1. if the electron later absorbs a photon with energy Δe Δ e, the electron returns to the n = 3 n = 3 shell (we examined the bohr model previously). figure 8.6.1 8.6. 1: an electron transition from the n = 3 n = 3 to the n = 2 n = 2 shell of a hydrogen atom. These internal electron transitions give rise to the emission of “characteristic” x radiation which, because of its short wavelength, has extremely high “penetrating” power. [since an electron beam is used to generate x rays, the x ray tube has to be evacuated: to dissipate the energy flux arriving at the target, the anode support (onto.

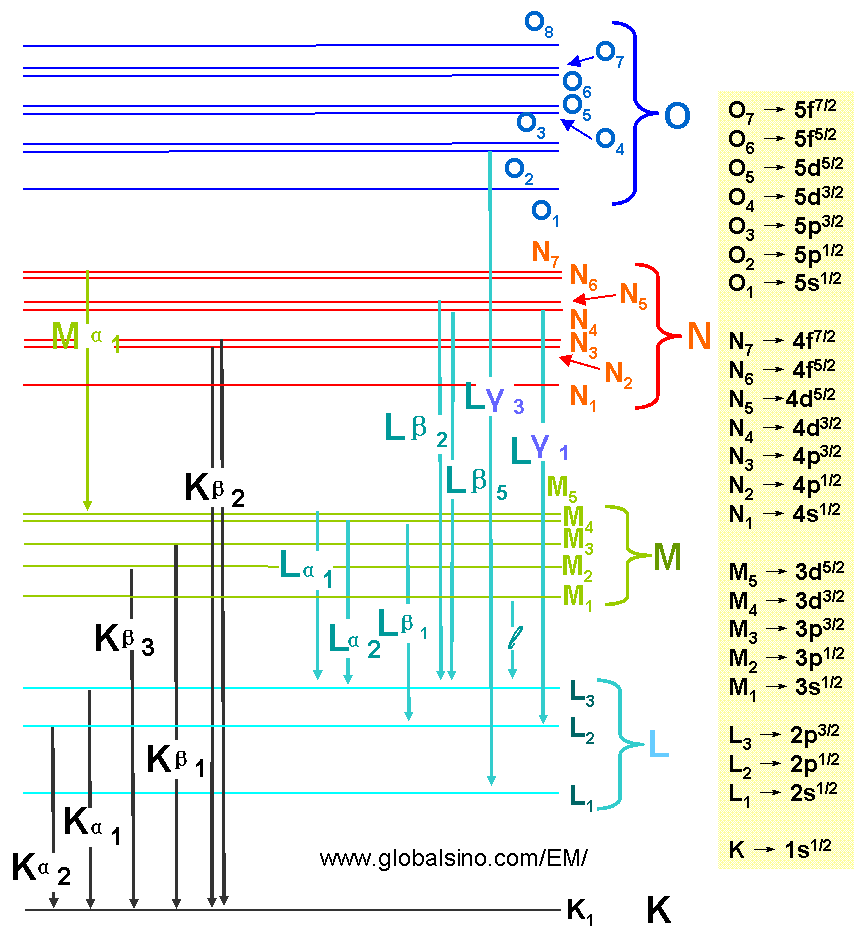
X Ray Detection And Analysis Ppt Video Online Download The bohr model of this process is shown in figure 8.6.1 8.6. 1. if the electron later absorbs a photon with energy Δe Δ e, the electron returns to the n = 3 n = 3 shell (we examined the bohr model previously). figure 8.6.1 8.6. 1: an electron transition from the n = 3 n = 3 to the n = 2 n = 2 shell of a hydrogen atom. These internal electron transitions give rise to the emission of “characteristic” x radiation which, because of its short wavelength, has extremely high “penetrating” power. [since an electron beam is used to generate x rays, the x ray tube has to be evacuated: to dissipate the energy flux arriving at the target, the anode support (onto. Figure 30.23 a characteristic x ray is emitted when an electron fills an inner shell vacancy, as shown for several transitions in this approximate energy level diagram for a multiple electron atom. characteristic x rays are labeled according to the shell that had the vacancy and the shell from which the electron came. Figure \(\pageindex{2}\): a characteristic x ray is emitted when an electron fills an inner shell vacancy, as shown for several transitions in this approximate energy level diagram for a multiple electron atom. characteristic x rays are labeled according to the shell that had the vacancy and the shell from which the electron came.

2 A Specimen Atom Characteristic X Rays The Electron Transition Figure 30.23 a characteristic x ray is emitted when an electron fills an inner shell vacancy, as shown for several transitions in this approximate energy level diagram for a multiple electron atom. characteristic x rays are labeled according to the shell that had the vacancy and the shell from which the electron came. Figure \(\pageindex{2}\): a characteristic x ray is emitted when an electron fills an inner shell vacancy, as shown for several transitions in this approximate energy level diagram for a multiple electron atom. characteristic x rays are labeled according to the shell that had the vacancy and the shell from which the electron came.

Comments are closed.