Planning Clinical Trial In Usa Here Is What You Should Know
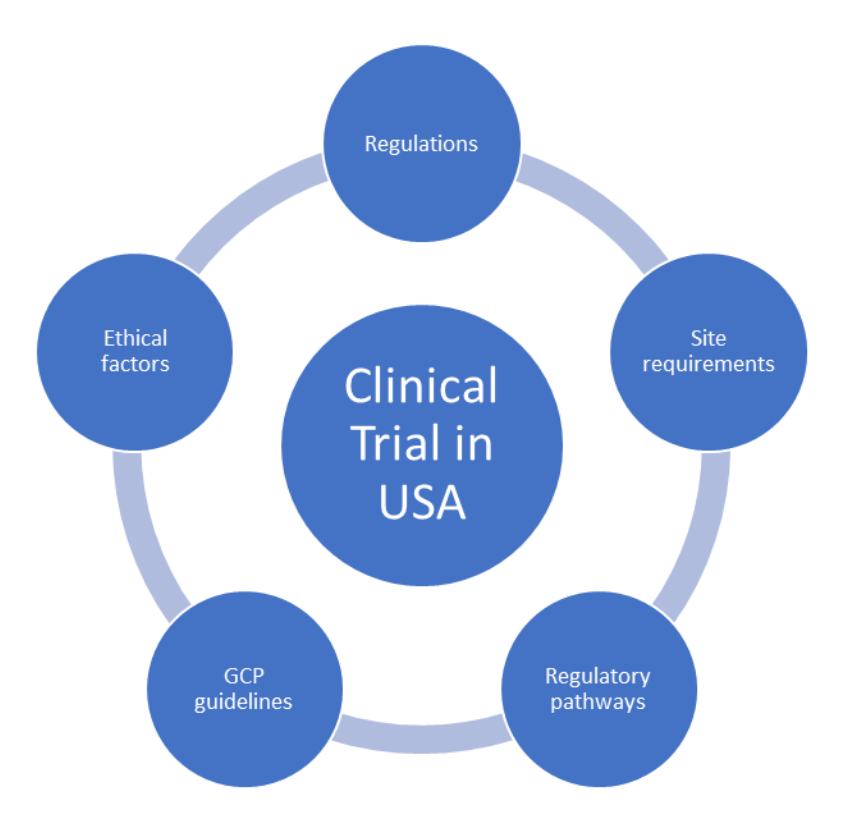
Planning Clinical Trial In Usa Here Is What You Should Know What you need to know to conduct a clinical trial in the united states. 1. regulations. sponsors, investigators, or cros planning to carry out clinical trials in the u.s. need to comply with. Here is what you should know. the united states has always been and remains to be the leading place for the conduct of clinical trials. according to clinicaltrials.gov, the largest clinical trials registry, 32% of registered clinical trials were conducted in the u.s. as of may 2022 (1). factors such as the availability of qualified healthcare.

Planning Clinical Trial In Usa Here Is What You Should Know By Clinical trials are conducted for many reasons: to determine whether a new drug or device is safe and effective for people to use. to study different ways to use standard treatments or current. Clinical trials guidance documents. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice and human subject protection. Clinical research includes all research that involves people. types of clinical research include: epidemiology, which improves the understanding of a disease by studying patterns, causes, and effects of health and disease in specific groups. behavioral, which improves the understanding of human behavior and how it relates to health and disease. These trials follow a specific study plan, called a protocol, that is developed by the researcher or manufacturer. before a clinical trial begins, researchers review prior information about the.

Planning Clinical Trial In Usa Here Is What You Should Know Clinical research includes all research that involves people. types of clinical research include: epidemiology, which improves the understanding of a disease by studying patterns, causes, and effects of health and disease in specific groups. behavioral, which improves the understanding of human behavior and how it relates to health and disease. These trials follow a specific study plan, called a protocol, that is developed by the researcher or manufacturer. before a clinical trial begins, researchers review prior information about the. A clinical trial is a research study that involves people like you. researchers conduct clinical trials to find new or better ways to prevent, detect, or treat health conditions. often, researchers want to find out if a new test, treatment, or preventive measure is safe and effective. tests can include ways to screen for, diagnose, or prevent a. Clinical research is the study of health and illness in people. there are two main types of clinical research: observational studies and clinical trials. read and share this infographic (pdf, 317k) to learn why researchers do different kinds of clinical studies. observational studies monitor people in normal settings.

Comments are closed.