Phrma Principles On Diversity In Conduct Of Clinical Trials Executive

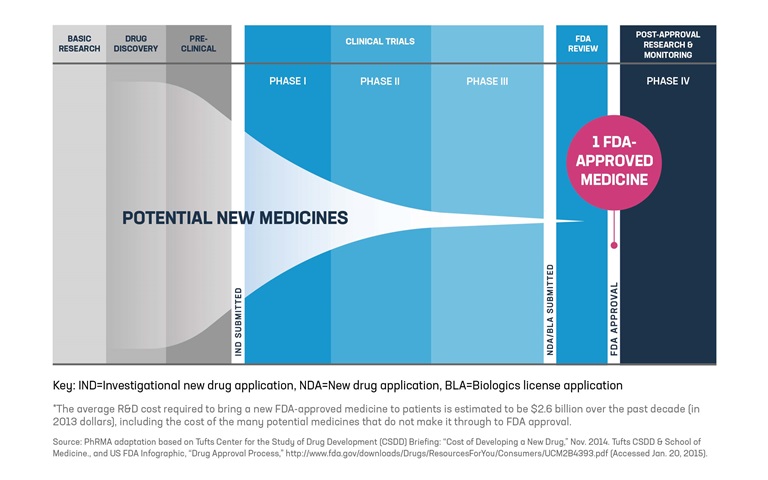
Phrma Principles On Diversity In Conduct Of Clinical Trials Executive Share this. in october 2020, phrma added a new chapter on enhancing diversity in clinical trial participation its principles on conduct in clinical trials. with this addition, phrma member companies commit to continuing to work with patients, patient advocacy groups, regulatory authorities, healthcare practitioners, academics, and policymakers. Phrma principles on conduct of clinical trials. developing new therapies to treat disease and to improve quality of life is a long and complex process. a critical part of that process is clinical research, the study of a pharmaceutical product in humans. without clinical research studies, no new medicines could be made available to patients.

Principles On Conduct Of Clinical Trials Phrma A recent report outlines five critical strategies for enhancing diversity in clinical trials and is based on more than a year of research and feedback from more than 500 stakeholders across 150 organizations. five key strategies: create a network of clinical trial sites in underserved communities. develop a diverse pool of investigators and staff. Phrma’s recently published principles on clinical trial diversity reinforce phrma member companies’ efforts and reflect their voluntary commitment to enhance diversity in future clinical trials. 9 phrma’s principles state that sponsors and investigators should consider the incidence, prevalence, and severity of the condition or disease in various populations, as well as other prognostic. "the industry's new clinical trial diversity principles are an important step toward greater health equity," said stephen j. ubl, president and chief executive officer of phrma. "we are addressing. In october, phrma’s board approved a new section called commitment to enhancing diversity in clinical trial participation, effective april 14, 2021. the other principles have been in effect since june 1, 2015. in the new section, phrma notes, “enhancing meaningful representation of diverse participants in clinical trials would help provide.
Clinical Trials Phrma "the industry's new clinical trial diversity principles are an important step toward greater health equity," said stephen j. ubl, president and chief executive officer of phrma. "we are addressing. In october, phrma’s board approved a new section called commitment to enhancing diversity in clinical trial participation, effective april 14, 2021. the other principles have been in effect since june 1, 2015. in the new section, phrma notes, “enhancing meaningful representation of diverse participants in clinical trials would help provide. “the industry’s new clinical trial diversity principles are an important step toward greater health equity,” said stephen j. ubl, president and chief executive officer of phrma. “we are addressing issues of mistrust and working to reduce systemic issues that deter communities of color from participating in clinical trials, so that those patients who want to participate, can.”. The phrma principles focus on four main areas: building trust and acknowledging the historic mistrust of clinical trials within black and brown communities, reducing barriers to clinical trial access, using real world data to enhance information on diverse populations beyond product approval and enhancing information about diversity and inclusion in clinical trial participation.

Clinical Trials Phrma “the industry’s new clinical trial diversity principles are an important step toward greater health equity,” said stephen j. ubl, president and chief executive officer of phrma. “we are addressing issues of mistrust and working to reduce systemic issues that deter communities of color from participating in clinical trials, so that those patients who want to participate, can.”. The phrma principles focus on four main areas: building trust and acknowledging the historic mistrust of clinical trials within black and brown communities, reducing barriers to clinical trial access, using real world data to enhance information on diverse populations beyond product approval and enhancing information about diversity and inclusion in clinical trial participation.
Us Phrma Announces Clinical Trial Diversity Principles

Comments are closed.