P Orbitals In Bonding Antibonding Mo Molecular Orbital Diagram O
Molecular Orbital Diagrams Explained Bond order is the amount of bonds formed between two atoms. for example, two bonds are formed between oxygen atoms, so the bond order is 2. the following is the equation to find bond order. 1 2 (electrons in bonding molecular orbitals electrons in antibonding molecular orbitals) bond order gives information about bond length and strength. This chemistry video tutorial provides a basic introduction into molecular orbital theory. it describes the formation of bonding and antibonding molecular o.
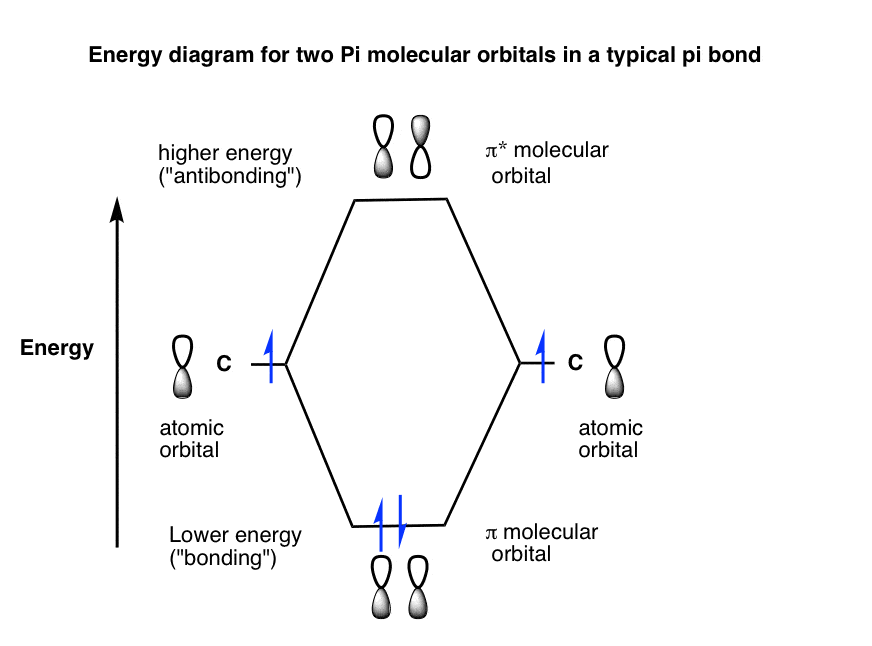
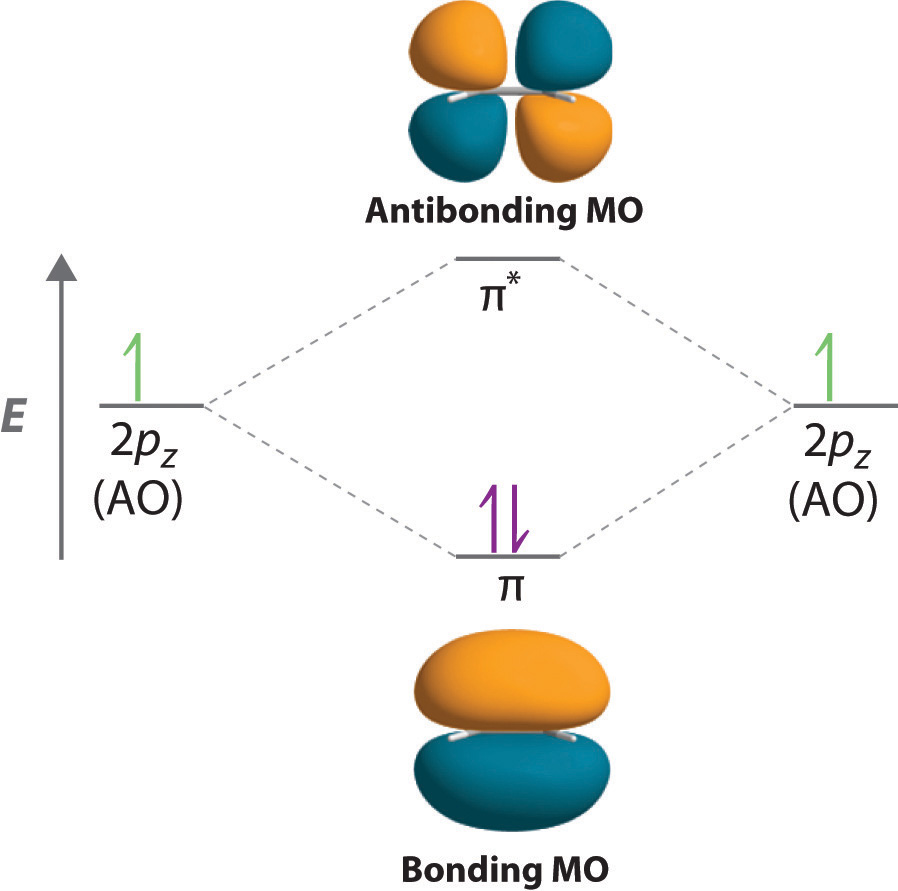
Molecular Orbital Diagram For A Simple Pi Bond вђ Bonding And Molecular orbital diagrams. this scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion h 2 . atomic valence electrons (shown in boxes on the left and right) fill the lower energy molecular orbitals before the higher ones, just as is the case for atomic. Molecular orbital theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals (s, p, d …) and hybrid orbitals (sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds. The side by side overlap of two p orbitals gives rise to a pi (π) bonding molecular orbital and a π* antibonding molecular orbital, as shown in figure 8.31. in valence bond theory, we describe π bonds as containing a nodal plane containing the internuclear axis and perpendicular to the lobes of the p orbitals, with electron density on either side of the node. Figure 1.19 a molecular orbital description of the c–c π bond in ethylene. the lower energy, π bonding mo results from an additive combination of p orbital lobes with the same algebraic sign and is filled. the higher energy, π antibonding mo results from a subtractive combination of p orbital lobes with opposite algebraic signs and is.

P Orbitals In Bonding Antibonding Mo Molecular Orbital The side by side overlap of two p orbitals gives rise to a pi (π) bonding molecular orbital and a π* antibonding molecular orbital, as shown in figure 8.31. in valence bond theory, we describe π bonds as containing a nodal plane containing the internuclear axis and perpendicular to the lobes of the p orbitals, with electron density on either side of the node. Figure 1.19 a molecular orbital description of the c–c π bond in ethylene. the lower energy, π bonding mo results from an additive combination of p orbital lobes with the same algebraic sign and is filled. the higher energy, π antibonding mo results from a subtractive combination of p orbital lobes with opposite algebraic signs and is. Valence bond theory. molecular orbital theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals (s, p, d …) and hybrid orbitals (sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ. A bonding orbital is formed when two atoms approach each other and the overlap between atomic orbitals results in constructive interference. the constructive interference causes the electron density between the two atoms to increase. this lowers the kinetic and potential energy of 1 or 2 electrons in the resulting bonding orbital.

Comments are closed.