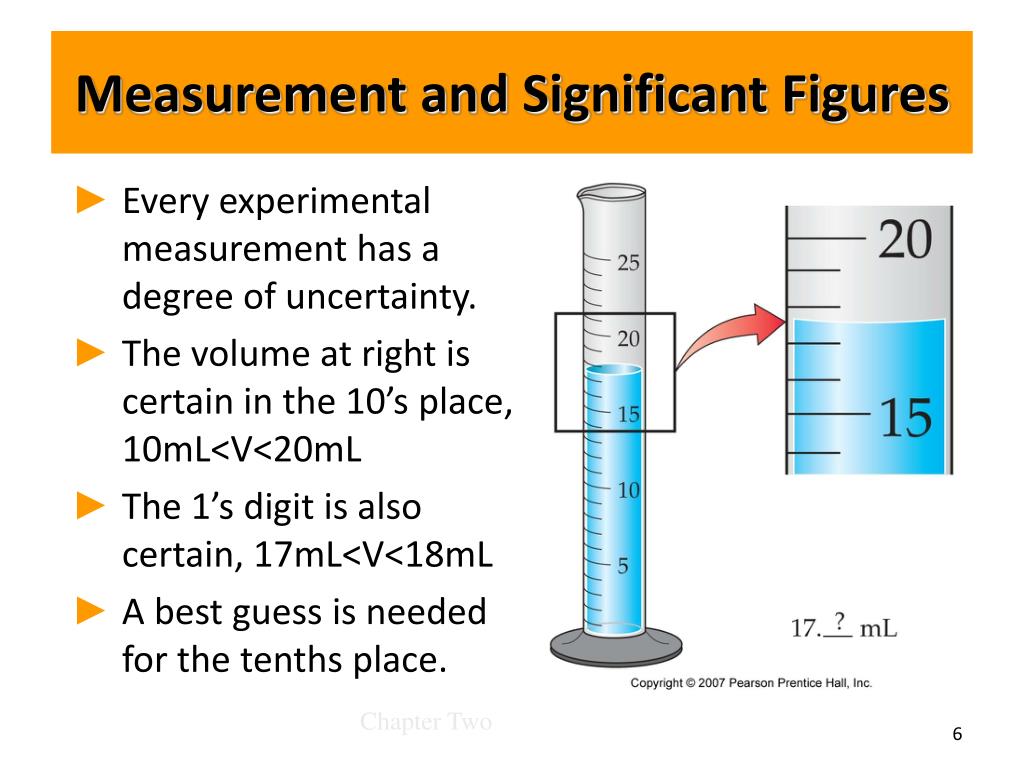
Measurement And Significant Figures
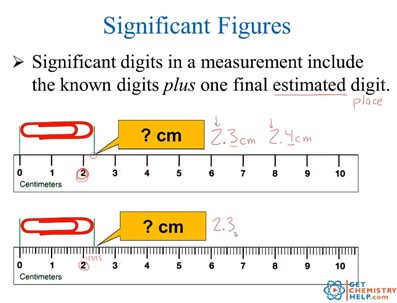
Ppt Significant Figures And Scientific Notation Powerpoint The rules for deciding which digits in a measurement are significant are as follows: rule 1: all nonzero digits in a measurement are significant. 237 has three significant figures. 1.897 has four significant figures. rule 2: zeros that appear between other nonzero digits (i.e., " middle zeros ") are always significant. The measurement taken with the top ruler contains 3 significant figures, because the visible graduations allow for a second certain digit to be known, and one uncertain digit must again be recorded. because the top ruler allows for a measurement with an additional significant figure, the top ruler is a superior ruler for measuring lengths.
Chemistry Lesson Significant Digits Measurements Get Chemistry Help Solution. the calculator answer is 2,085.5688, but we need to round it to five significant figures. because the first digit to be dropped (in the tenths place) is greater than 5, we round up to 2,085.6. the calculator gives 1,125 as the answer, but we limit it to three significant figures. So 1300 could have two, three, or four significant figures. to avoid this ambiguity, we should write 1300 in scientific notation as 1.3 x 10 3, 1.30 x 10 3, or 1.300 x 10 3, depending on whether it has two, three, or four significant figures. zeros are significant except when they serve only as placeholders. The rules for determining the number of significant figures are as follows: all nonzero digits are significant. for example, the value 211.8 has four significant figures. all zeros that are found between nonzero digits are significant. thus, the number 20,007, with three 0s between the 2 and 7, has a total of five significant figures. These measurements are quite accurate because they are very close to the correct value of 11.0 inches. in contrast, if you had obtained a measurement of 12 inches, your measurement would not be very accurate. figure 1.3.1 1.3. 1: a double pan mechanical balance is used to compare different masses.

Precision Accuracy Measurement And Significant Figures Youtube The rules for determining the number of significant figures are as follows: all nonzero digits are significant. for example, the value 211.8 has four significant figures. all zeros that are found between nonzero digits are significant. thus, the number 20,007, with three 0s between the 2 and 7, has a total of five significant figures. These measurements are quite accurate because they are very close to the correct value of 11.0 inches. in contrast, if you had obtained a measurement of 12 inches, your measurement would not be very accurate. figure 1.3.1 1.3. 1: a double pan mechanical balance is used to compare different masses. The significant figures of a number are those digits that carry meaning contributing to its precision. thus the number of significant digits depends on the least count of the measuring instrument. all the certain digits and the one uncertain digit are called the significant figures in the measured value. rules to find significant figures specifically, the rules for identifying significant. 0.00034 has 2 significant figures (3 and 4) if the resolution is 0.00001. zeros to the right of the last non zero digit (trailing zeros) in a number with the decimal point are significant if they are within the measurement or reporting resolution. 1.200 has four significant figures (1, 2, 0, and 0) if they are allowed by the measurement resolution.

Comments are closed.