Mdsap Medical Device Single Audit Program
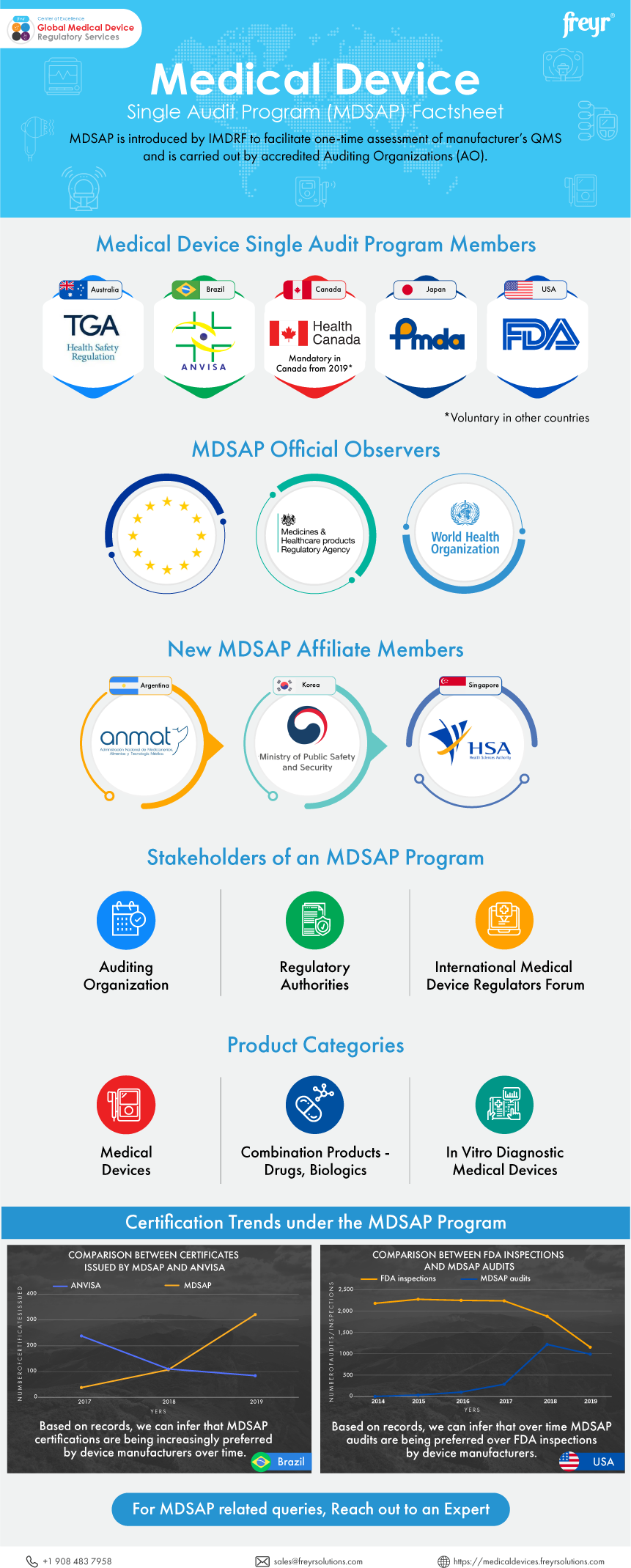
Medical Device Single Audit Program Mdsap Factsheet Freyr Med DIAGNOS must undergo thorough statutory quality compliance audits under the Medical Device Single Audit Program (MDSAP) MDSAP is a comprehensive approach to quality management systems auditing The UC Davis Department of Biomedical Engineering is launching a new nine-month master’s degree program in medical device development at Aggie Square, the expansive innovation district the university

Medical Device Single Audit Program Mdsap Explained EPA’s in-house watchdog is launching an audit of the agency’s use of tens of millions of dollars in community air quality monitoring grants set aside by Congress in recent years, according to Treating Obesity: Not for a Lack of Trying Obesity specialists explain the societal, economic, and cultural barriers that prevent patients from seeking medical intervention for the treatment of The Montgomery Internal Medicine Residency Program provides high-caliber graduate medical education for trainees seeking diverse clinical experiences in both inpatient and outpatient settings, and in A new methodology that allows for the categorization and organization of single-cell data has been launched It can be used to create a harmonized dataset for the study of human health and disease

Comments are closed.