Management Of Hiv In Pregnant Women

Management Of Hiv In Pregnant Women Recommendations. the american college of obstetricians and gynecologists makes the following recommendations: established and ongoing research has shown that treatment of human immunodeficiency virus (hiv) infected pregnant women with combined antiretroviral therapy (cart) can achieve a 1–2% or lower risk of mother to child transmission if maternal viral loads of 1,000 copies ml or less can. The guidelines are aimed at clinical professionals directly involved with, and responsible for, the care of pregnant women living with hiv. the 2018 guidelines have identified significant developments that have either led to a change in recommendation or a change in the strength of recommendation.

Hiv And Getting Pregnant Shecares The study of hiv during pregnancy holds great significance because many women are first diagnosed with hiv during pregnancy. similarly, it is equally important in cases where one or both partners are hiv positive and wish to conceive. during the recent years, universal hiv prenatal testing, antiretroviral therapy (art), scheduled cesarean delivery for hiv positive women with elevated viral. Yes, all pregnant people with hiv should take hiv medicines throughout pregnancy for three reasons: 1) their own health, 2) to prevent perinatal transmission of hiv to the baby, and 3) to prevent hiv transmission to sexual partners. perinatal transmission of hiv means passing hiv from the mother or birthing parent to their child. Abstract. human immunodeficiency virus (hiv) is an infection with a global prevalence and currently no cure or vaccine. women living with hiv who become pregnant or who acquire the virus during pregnancy are at risk of both maternal and perinatal morbidity and mortality mainly if the virus is poorly controlled. In general, the panel on treatment of hiv during pregnancy and prevention of perinatal transmission (the panel) recommends that people who are already on fully suppressive arv regimens when pregnancy occurs should continue with those regimens, unless they are receiving an arv drug or arv regimen that is not recommended for use in nonpregnant.

Hiv In Pregnancy Abstract. human immunodeficiency virus (hiv) is an infection with a global prevalence and currently no cure or vaccine. women living with hiv who become pregnant or who acquire the virus during pregnancy are at risk of both maternal and perinatal morbidity and mortality mainly if the virus is poorly controlled. In general, the panel on treatment of hiv during pregnancy and prevention of perinatal transmission (the panel) recommends that people who are already on fully suppressive arv regimens when pregnancy occurs should continue with those regimens, unless they are receiving an arv drug or arv regimen that is not recommended for use in nonpregnant. Panel’s recommendations; the plasma hiv rna levels of pregnant people with hiv should be monitored at the initial antenatal visit with a review of prior hiv rna levels (ai), 2 to 4 weeks after initiating (or changing) antiretroviral therapy (art) (bi), monthly until rna levels are undetectable (biii), and then at least every 3 months during pregnancy (biii). January 31, 2024. the panel on treatment of hiv during pregnancy and prevention of perinatal transmission (the panel) has updated text and references throughout the guidelines to include new data and publications where relevant and to incorporate gender inclusive language. these changes are highlighted in yellow in the pdf version of the.

35 Management Of Hiv Positive Pregnancy Panel’s recommendations; the plasma hiv rna levels of pregnant people with hiv should be monitored at the initial antenatal visit with a review of prior hiv rna levels (ai), 2 to 4 weeks after initiating (or changing) antiretroviral therapy (art) (bi), monthly until rna levels are undetectable (biii), and then at least every 3 months during pregnancy (biii). January 31, 2024. the panel on treatment of hiv during pregnancy and prevention of perinatal transmission (the panel) has updated text and references throughout the guidelines to include new data and publications where relevant and to incorporate gender inclusive language. these changes are highlighted in yellow in the pdf version of the.
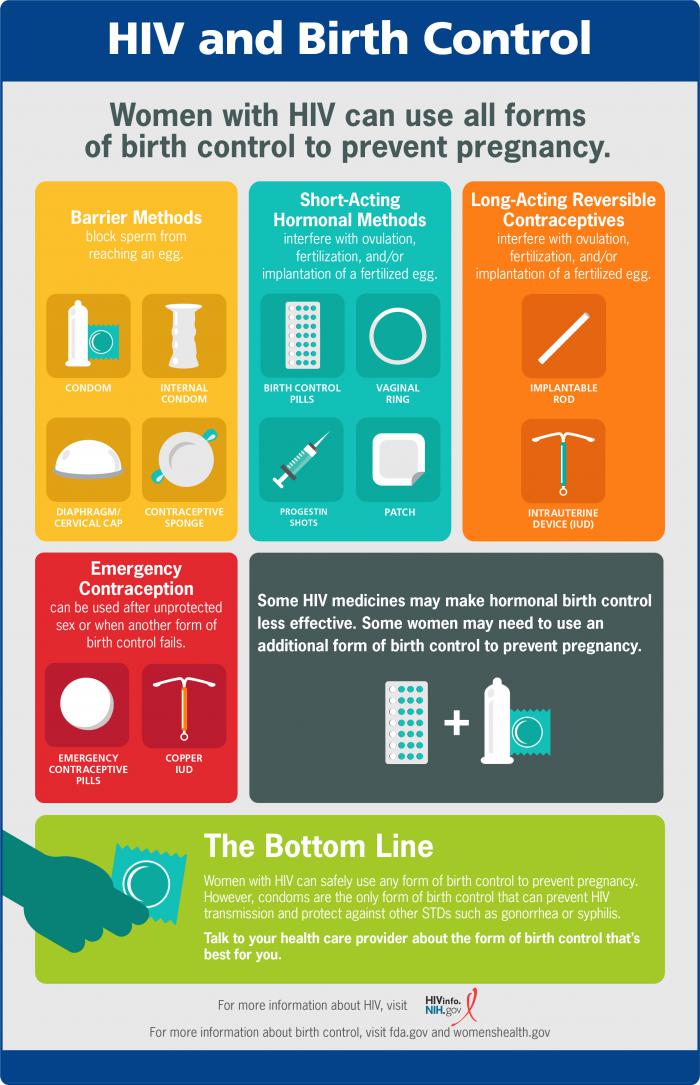
Hiv And Women S Health Issues Hiv Gov

Comments are closed.