Lab 2 Investigation Of Density

Lab 2 Investigation Of Density Youtube Density is determined by dividing the mass of a substance by its volume: density = mass volume (2.1) (2.1) d e n s i t y = m a s s v o l u m e. the units of density are commonly expressed as g cm 3 for solids, g ml for liquids, and g l for gases. density is also an intensive property of matter. Mass, volume, and density v5 (two lab periods) 5 figure 2. reading a graduated cylinder. in a second example, shown in figure 3, notice the graduations at 2 and 3 ml with nine markings between them, each corresponding to 0.1 ml. in addition, notice how this meniscus is not on a line but rather between 2.6 and 2.7 ml.

Lab 02 Density And The Scientific Method 1 Pdf Name Section Number It is important to note that liquid water has a density of approximately 1.0 g ml. so at room temperature, 50.0 ml of water has a mass of almost exactly 50.0 g. two factors have an effect on the density of water: temperature will have a small effect on the density. for water, density increases as temperature decreases. 15 density science experiments. by amy cowen on march 13, 2024 8:00 am. use these free science lessons, experiments, and activities to teach k 12 students about density. sometimes students wrongly think that an object's density is the same as its weight or its mass. instead, density refers to an object's mass in a given volume. D=m v. density equation. accuracy. a measure of how close an experimental value is to the actual value. precision. the reproducibility of the measurement or how closely the measurements agree with each other. i would rather be accurate because its definition is how well ou hit our target, not that you can hit a spot over and over again, he just. Lab 1 (weeks 1 & 2) introducing measurements in the laboratory; lab 2 (week 3) the density of liquids and solids. learning outcomes; reading; attribution; lab 3 (week 4) nomenclature of ionic compounds; lab 4 (week 5) chemical formlua determination; lab 5 (week 6) types of reactions; lab 6 (week 7) mole ratios and reaction stoichiometry.

Lab 2 Density Pdf Chemistry 22 Experiment 2 Density Introduction D=m v. density equation. accuracy. a measure of how close an experimental value is to the actual value. precision. the reproducibility of the measurement or how closely the measurements agree with each other. i would rather be accurate because its definition is how well ou hit our target, not that you can hit a spot over and over again, he just. Lab 1 (weeks 1 & 2) introducing measurements in the laboratory; lab 2 (week 3) the density of liquids and solids. learning outcomes; reading; attribution; lab 3 (week 4) nomenclature of ionic compounds; lab 4 (week 5) chemical formlua determination; lab 5 (week 6) types of reactions; lab 6 (week 7) mole ratios and reaction stoichiometry. Video 1: virtual chemistry experiment: exploring density part 1 on the science classroom channel (published 9 2 2020) after this introduction, students use the phet simulation, states of matter basic, to observe solids, liquids, and gases at the particulate level (image 1). then, students answer a few analysis questions to deepen their. Without an account, gizmos can be viewed for just 5 minutes each per day. with a scale to measure mass, a graduated cylinder to measure volume, and a large beaker of liquid to observe flotation, the relationship between mass, volume, density, and flotation can be investigated. the density of the liquid in the beaker can be adjusted, and a.
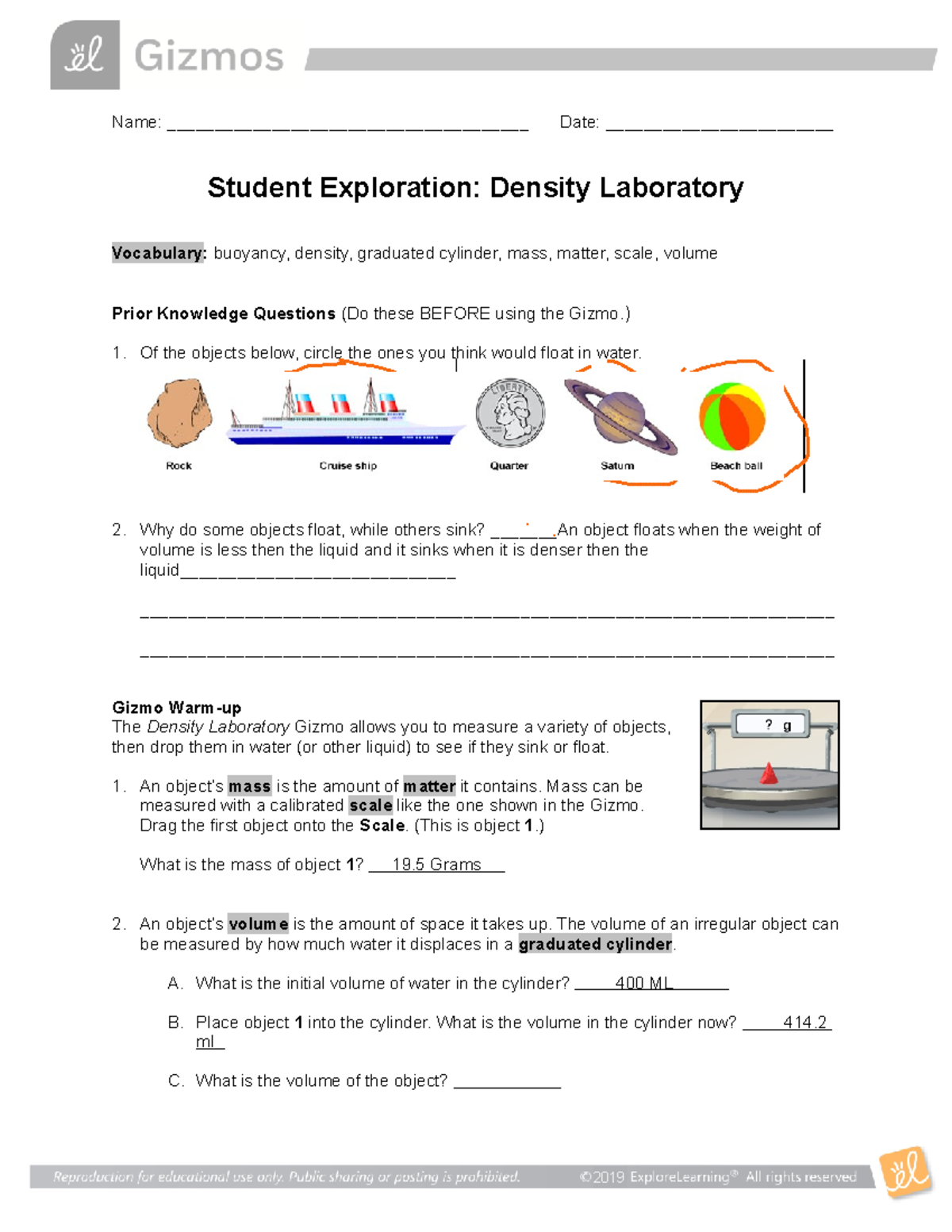
Density Lab 2 F Name Date Video 1: virtual chemistry experiment: exploring density part 1 on the science classroom channel (published 9 2 2020) after this introduction, students use the phet simulation, states of matter basic, to observe solids, liquids, and gases at the particulate level (image 1). then, students answer a few analysis questions to deepen their. Without an account, gizmos can be viewed for just 5 minutes each per day. with a scale to measure mass, a graduated cylinder to measure volume, and a large beaker of liquid to observe flotation, the relationship between mass, volume, density, and flotation can be investigated. the density of the liquid in the beaker can be adjusted, and a.
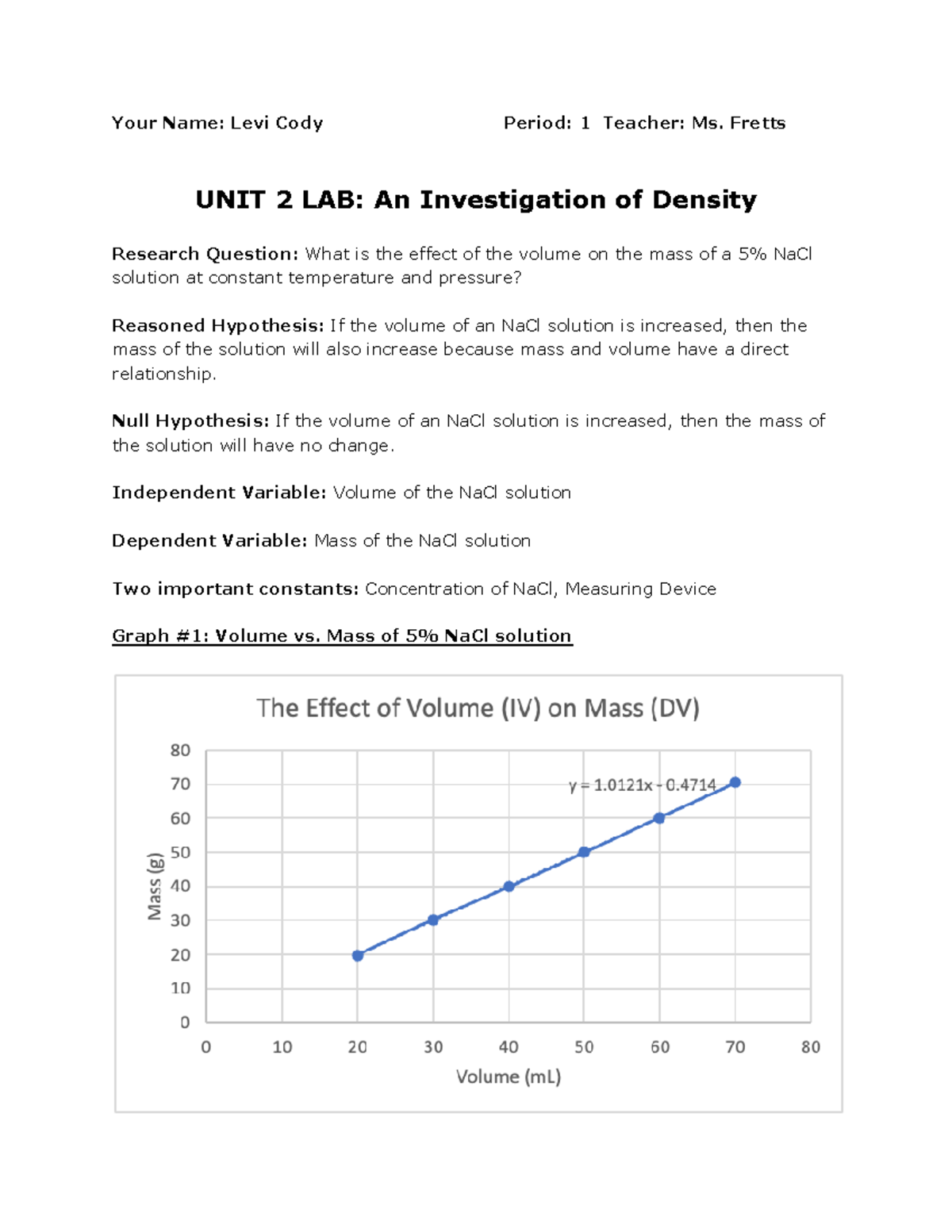
An Investigation Of Density Lab Report Your Name Levi Cody Period 1

Comments are closed.