Issues With Covid 19 Rapid Tests In Nursing Homes

Nevada Halts Use Of Rapid Coronavirus Tests In Nursing Homes The New Alberta: alberta has been offering rapid testing at long term care homes in edmonton and other communities using mobile testing units. this is an expansion of the province's rapid covid 19 testing program, which has tested over 1,000 albertans. in a matter of hours, 76 people were notified of their positive results. Around the world, residential care settings such as nursing homes and long term care facilities (ltcfs) have seen repeated covid 19 outbreaks and been a conspicuous source of covid 19 morbidity and mortality.1, 2, 3 age is an independent, nonmodifiable risk factor for covid 19–related morbidity; poor prognostic outcomes increase with advancing age, and mortality rates of up to 15% have been.

Covid Testing Hell Devices Given To Nursing Homes Bring New Problems An especially important setting for surveillance testing is skilled nursing facilities, whose residents and staff made up less than 2% of the u.s. population but more than 20% of covid 19. Abstract. older adults in nursing homes are at greatest risk of morbidity and mortality from sars cov 2 infection. nursing home residents constituted one third to more than half of all deaths during the early waves of the covid 19 pandemic. following this, widespread adaptation of infection prevention and control measures and the supply and use. Performing facility wide sars cov 2 testing in nursing homes. sars cov 2 (covid 19) fact sheet: guidance – proposed use of point of care testing platforms for sars cov 2 (covid 19) [63 kb, 3 pages] cms. cms covid 19 faqs on medicare fee for service billing. cms clia post public health emergency (phe) guidance. cms clia faqs: post phe guidance. Introduction. covid 19 has caused unprecedented challenges in nursing homes. in this scoping review, we aimed to describe factors that contributed to the spread and mortality of covid 19 in nursing homes and provide an overview of responses that were implemented to try to overcome such challenges.
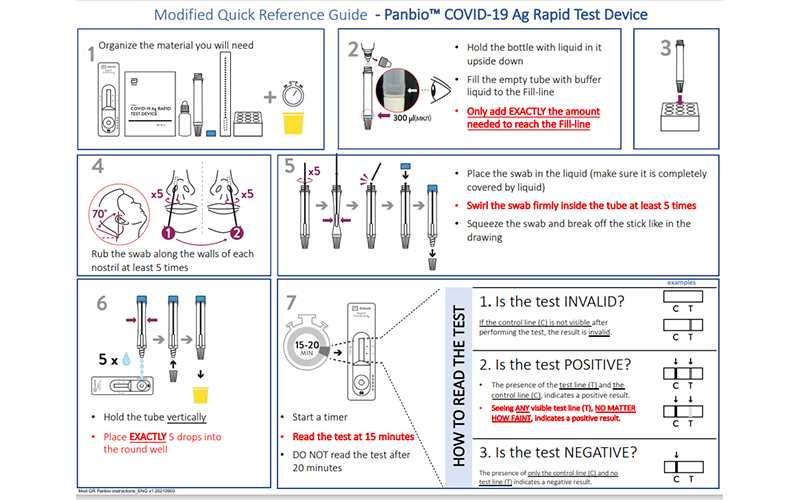
Rapid Antigen Detection Tests For Covid 19 Using And Reading Them Well Performing facility wide sars cov 2 testing in nursing homes. sars cov 2 (covid 19) fact sheet: guidance – proposed use of point of care testing platforms for sars cov 2 (covid 19) [63 kb, 3 pages] cms. cms covid 19 faqs on medicare fee for service billing. cms clia post public health emergency (phe) guidance. cms clia faqs: post phe guidance. Introduction. covid 19 has caused unprecedented challenges in nursing homes. in this scoping review, we aimed to describe factors that contributed to the spread and mortality of covid 19 in nursing homes and provide an overview of responses that were implemented to try to overcome such challenges. Covid 19: questions and answers (see “testing in nursing homes” section). if a resident or staff has signs or symptoms of covid 19 and the antigen test is positive, a confirmatory test (e.g., reverse transcriptase polymerase chain reaction (rt pcr) or different antigen test platform) is not recommended. a facility should immediately. Questions related to obtaining the instruments, test kits and all of the disposables for performing the test, payment issues for snf nf testing, or any other snf nf questions are outside the scope of the clia program and should be referred to the following mailbox: dnh [email protected]. 27.
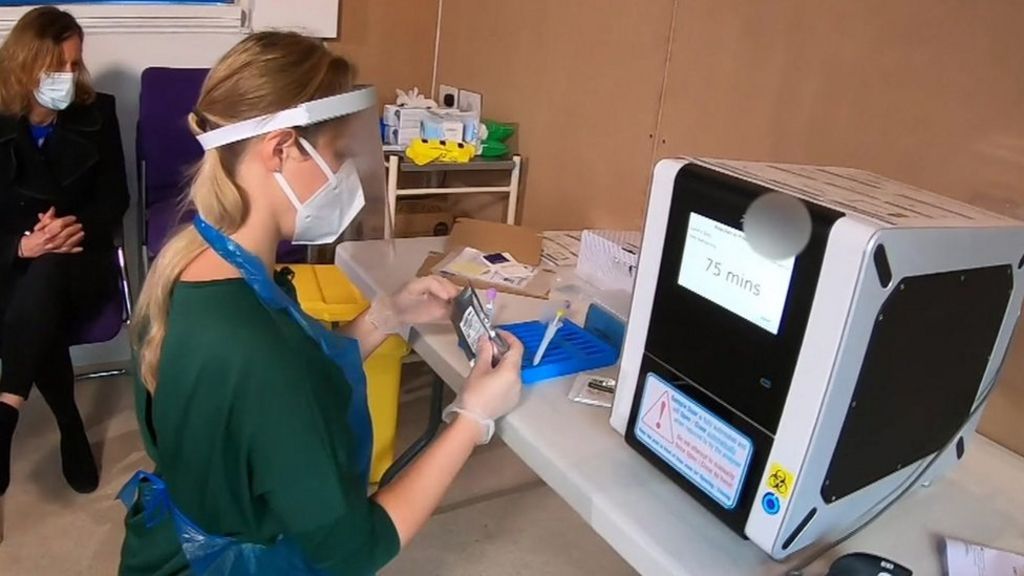
Coronavirus Rapid Tests In Care Homes A Game Changer Bbc News Covid 19: questions and answers (see “testing in nursing homes” section). if a resident or staff has signs or symptoms of covid 19 and the antigen test is positive, a confirmatory test (e.g., reverse transcriptase polymerase chain reaction (rt pcr) or different antigen test platform) is not recommended. a facility should immediately. Questions related to obtaining the instruments, test kits and all of the disposables for performing the test, payment issues for snf nf testing, or any other snf nf questions are outside the scope of the clia program and should be referred to the following mailbox: dnh [email protected]. 27.

Comments are closed.