Introduction To Pressure Force Area Units Atmospheric Gases Elevation Boiling Point

Introduction To Pressure Force Area Units Atmospheric Gasesођ This chemistry video tutorial provides a basic introduction to pressure. pressure is defined as force per unit area. 1 pascal equals 1 newton of force per. This relationship between force, area, and pressure is described by pascal's principle. q: how does elevation affect atmospheric pressure? as elevation increases, atmospheric pressure decreases. this is because there are fewer gas molecules above, exerting a smaller force per unit area. at higher elevations, the pressure is lower.
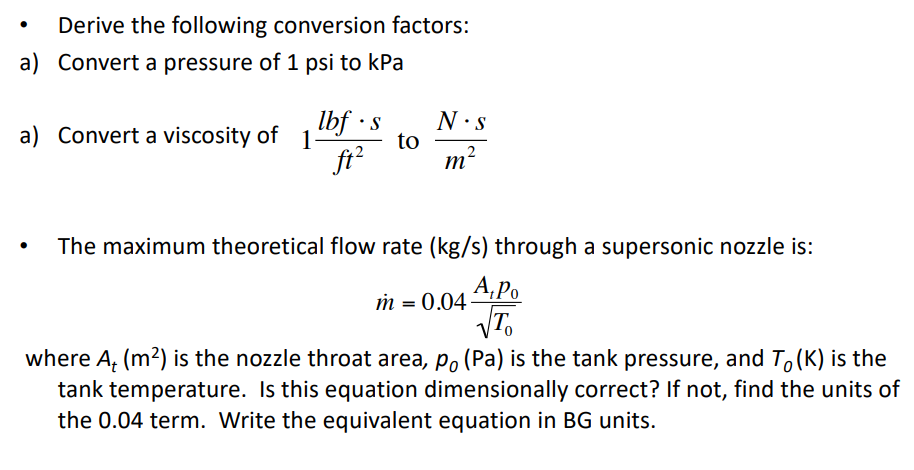
Introduction To Pressure Force Area Units Atmospheric 60 Off Chapter 1 contained a vast array of topics, from defining temperature and pressure, to describing atmospheric vertical structure and components. as a reminder, these were our learning goals: convert between temperature units of fahrenheit, celsius, and kelvin. use mathematical formulas to define atmospheric temperature, pressure, and density. As elevation increases, atmospheric pressure and boiling point decrease. boiling point is the point at which vapour pressure equals atmospheric pressure. in a liquid, some particles always have enough energy to escape to the gas phase. gaseous particles are also returning to the liquid. the vapour pressure is the pressure exerted by the gas when the amount of particles leaving the liquid. Atmospheric pressure is the force per unit area exerted by a body of air above a specified area (called an atmospheric column). it is expressed in several different systems of units, including millimeters (or inches) of mercury, pounds per square inch (psi), millibars (mb), or standard atmospheres. Boiling point. water boiling at 99.3 °c (210.8 °f) at 215 m (705 ft) elevation. the boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [1][2] and the liquid changes into a vapor. the boiling point of a liquid varies depending upon the surrounding environmental.
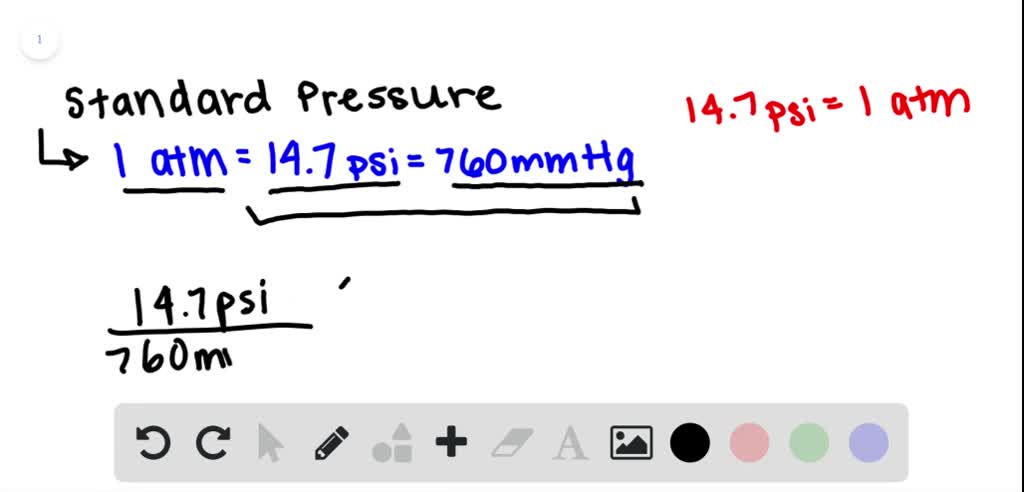
Introduction To Pressure Force Area Units Atmospheric 60 Off Atmospheric pressure is the force per unit area exerted by a body of air above a specified area (called an atmospheric column). it is expressed in several different systems of units, including millimeters (or inches) of mercury, pounds per square inch (psi), millibars (mb), or standard atmospheres. Boiling point. water boiling at 99.3 °c (210.8 °f) at 215 m (705 ft) elevation. the boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [1][2] and the liquid changes into a vapor. the boiling point of a liquid varies depending upon the surrounding environmental. The boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea level atmospheric pressure (760 mm [29.92 inches] of mercury). at sea level, water boils at 100° c (212° f). at higher altitudes the temperature of the boiling point is. The boiling point is the temperature at which a liquid boils. the liquid changes into a vapor and the vapor pressure of the liquid is the same as the external environment. the simple definition of boiling point is that it is the temperature at which a liquid boils. for example, the boiling point of water at sea level is 100 °c or 212 °f.

Comments are closed.