Indicaid Covid 19 Rapid Antigen Test By Phase Scientific вђ Rhino
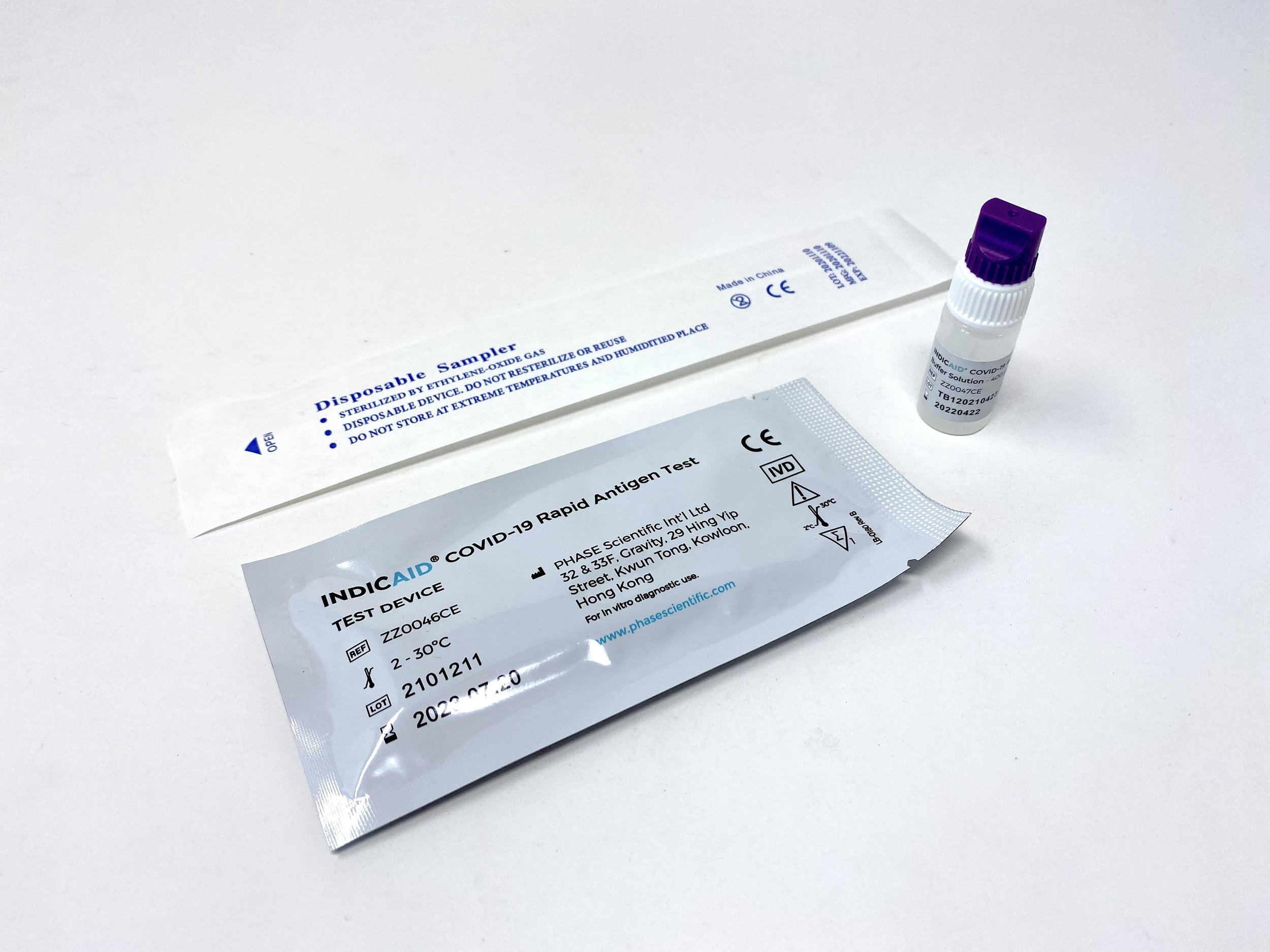
Indicaid Covid 19 Rapid Antigen Test By Phase Scientific в Instruction for use. read the results promptly in 20 minutes (results after 25 minutes should not be used) step 1. collect anterior nasal swab specimen. stir the swab into the buffer solution by twisting the swab back and forth 20 times. drop 3 drops of the buffer solution into the test device. Indicaid covid 19 antigen rapid at home test is an easy way to detect sars cov 2 nucleocapsid protein antigen. it only takes 4 steps and about 20 minutes to complete the test. the test is also non invasive. * purchase up to 1000 tests from our online store by clicking the link below. for orders over 1000 tests, please contact our sales team by.

Indicaid Covid 19 Rapid Antigen Test By Phase Scientific в Features. quick: with indicaid, users can expect quick results in just 20 minutes. easy: user friendly testing. no additional equipment or training is required. simple sampling: indicaid comes with intuitive vials to obtain self collected shallow nasal samples. bulk testing: our rapid covid 19 test allows for the aggregate collection of. The speedyswab™ rapid covid 19 flu a&b antigen self test is a lateral flow immunoassay intended for the qualitative detection and differentiation of sars cov 2, influenza a, and influenza b protein antigens. this test is authorized for non prescription home use with self collected anterior nasal swab specimens from individuals aged 14 years. Your sample(s) was tested for covid 19 using the indicaidtm covid 19 rapid antigen test. this fact sheet contains information to help you understand the risks and benefits of using this test for. Phase scientific international ltd (phase scientific), today announced that its indicaid™ covid 19 rapid antigen test (indicaid) received emergency use authorization (eua) from the u.s. food and drug administration (fda) on july 29, 2021. the fda authorized the test for professional use in point of care clia waived settings in the u.s. for.

Indicaid Poc Covid 19 Rapid Antigen Test Phase Scientif Your sample(s) was tested for covid 19 using the indicaidtm covid 19 rapid antigen test. this fact sheet contains information to help you understand the risks and benefits of using this test for. Phase scientific international ltd (phase scientific), today announced that its indicaid™ covid 19 rapid antigen test (indicaid) received emergency use authorization (eua) from the u.s. food and drug administration (fda) on july 29, 2021. the fda authorized the test for professional use in point of care clia waived settings in the u.s. for. The indicaid™ covid 19 rapid antigen test has been designed to minimize the likelihood of false positive test results. however, in the event of a false positive result, risks could include the. The indicaid® covid 19 rapid antigen at home test is authorized for non prescription home use with self collected shallow nasal samples from individuals aged 14 years or older, or adult collected.

Indicaid Covid 19 Rapid Antigen Test By Phase Scientific в The indicaid™ covid 19 rapid antigen test has been designed to minimize the likelihood of false positive test results. however, in the event of a false positive result, risks could include the. The indicaid® covid 19 rapid antigen at home test is authorized for non prescription home use with self collected shallow nasal samples from individuals aged 14 years or older, or adult collected.

Comments are closed.