How To Get Into A Clinical Trial

How To Get Into A Clinical Trial This is a searchable registry and results database of federally and privately supported clinical trials conducted in the united states and around the world. clinicaltrials.gov gives you information about a trial's purpose, who may participate, locations, and phone numbers for more details. this information should be used in conjunction with. 5—write and speak clearly. aside from strong technical skills for many jobs, you may also need to demonstrate above average written and verbal skills. this is important because clinical research is a cross functional, team oriented field. for most roles, you’ll be working in a team environment. when the job description states, “candidate.

Clinical Research Stony Brook Medicine Step 1: gather details about your cancer. if you decide to look for a clinical trial, you will need to know certain details about your cancer diagnosis and compare these details with the eligibility criteria of any trial that interests you. eligibility criteria are the requirements that must be met for you to join a clinical trial. Clinical trials are conducted for many reasons: to determine whether a new drug or device is safe and effective for people to use. to study different ways to use standard treatments or current. The clinical trial study protocol. the study protocol is the written plan for a clinical trial. it’s sent to the food and drug administration (fda) and to an institutional review board (irb) for approval before a new treatment can be studied in people. a protocol describes: why the clinical trial is being done. Pre screening. if you are considering participating in a clinical trial and want to see if you meet the eligibility criteria, often the first step is to go through an initial screening process. this is called pre screening. you’ll answer some questions about your health and medical history, typically in an online questionnaire or over the phone.
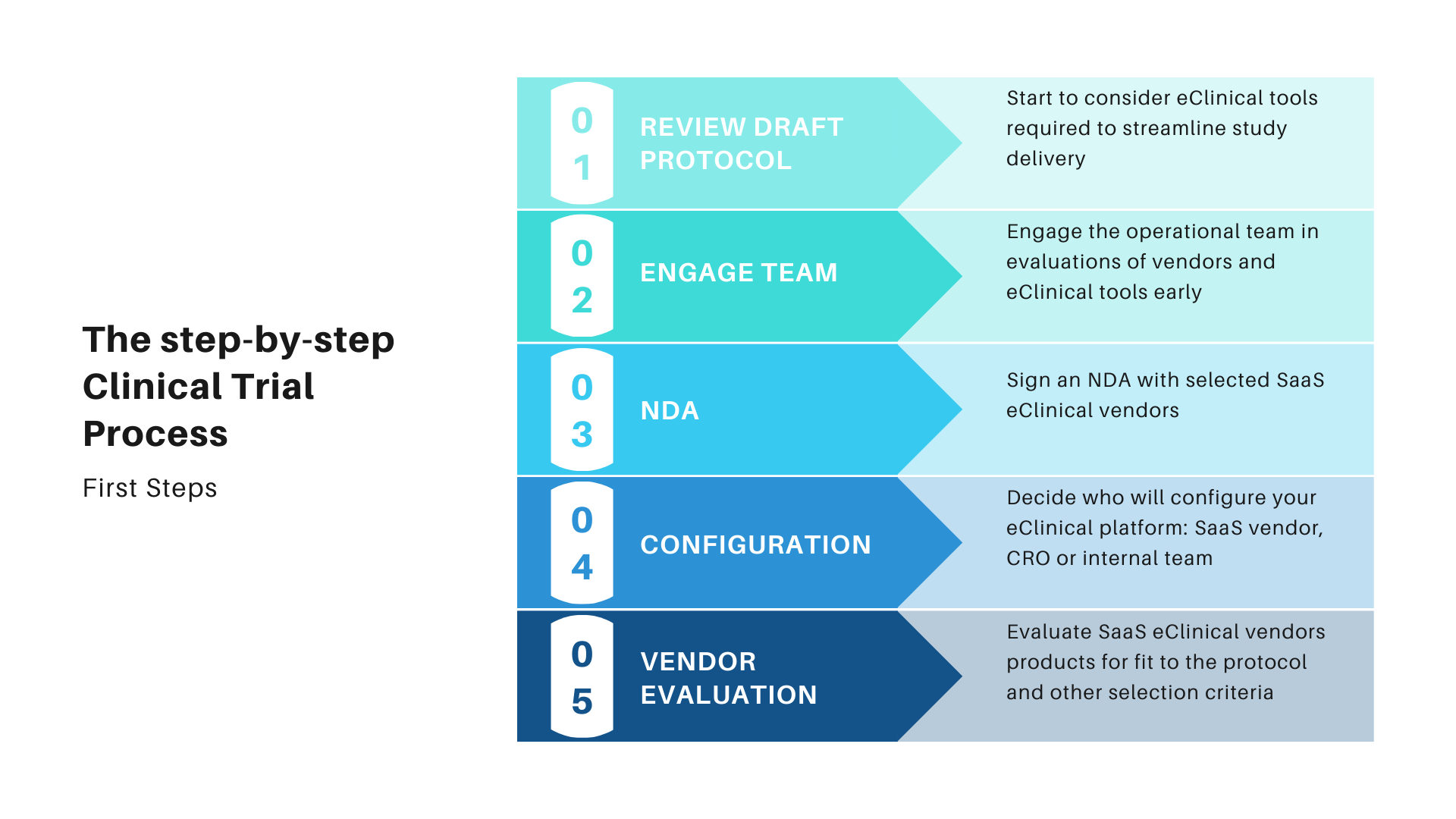
The Clincial Trial Process Step By Step Approach The clinical trial study protocol. the study protocol is the written plan for a clinical trial. it’s sent to the food and drug administration (fda) and to an institutional review board (irb) for approval before a new treatment can be studied in people. a protocol describes: why the clinical trial is being done. Pre screening. if you are considering participating in a clinical trial and want to see if you meet the eligibility criteria, often the first step is to go through an initial screening process. this is called pre screening. you’ll answer some questions about your health and medical history, typically in an online questionnaire or over the phone. The idea for a clinical trial often starts in the lab. after researchers test new treatments or procedures in the lab and in animals, the most promising treatments are moved into clinical trials. as new treatments move through a series of steps called phases, more information is gained about the treatment, its risks, and its effectiveness. Go to clinicaltrials.gov. click on the link, "search for clinical trials," on the home page. enter your search terms for example, a disease or intervention and a location: "heart attack.

Comments are closed.