How To Find The Number Of Protons Electrons Neutrons For Copper Cu

How To Find The Number Of Protons Electrons Neutrons For Copper Cu In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and neutrons for the element copper (cu). from the periodic. The number of electrons in an electrically neutral atom is the same as the number of protons in the nucleus. therefore, the number of electrons in neutral atom of copper is 29. each electron is influenced by the electric fields produced by the positive nuclear charge and the other (z – 1) negative electrons in the atom.
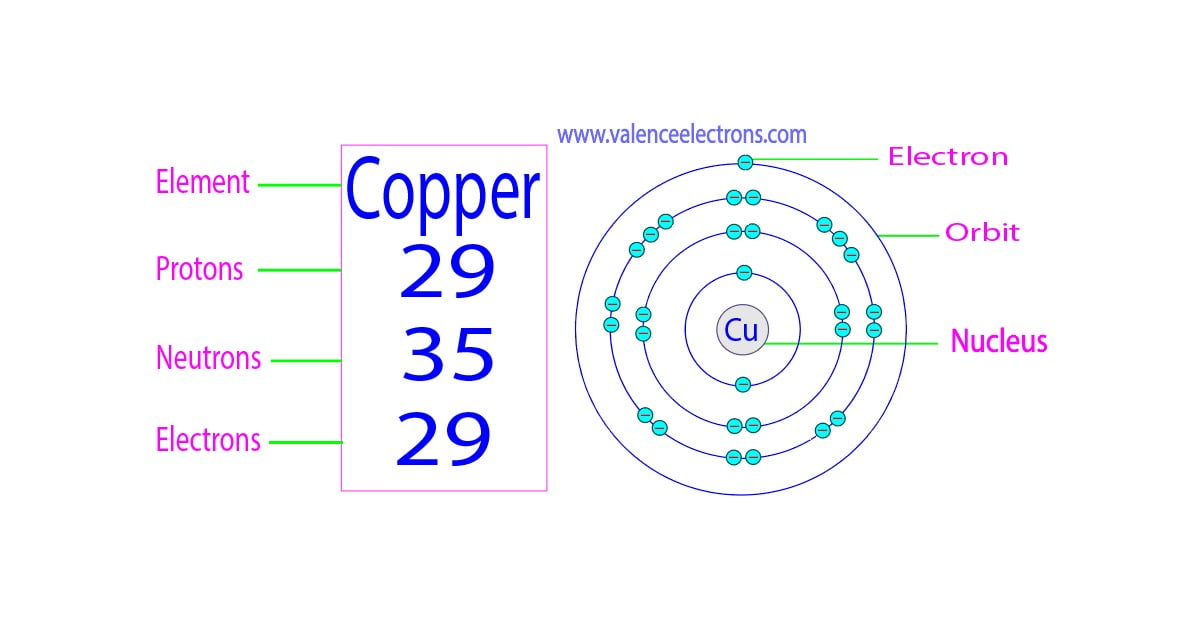
Copper Periodic Table Protons Neutrons And Electrons Elcho Table First, to find the number of protons, we need to realize that the neutral atom had 53 electrons because it is the additional one electron that makes it a 1 anion. now, because the atom has 53 electrons, it must also have 53 protons, and to find the number of neutrons we subtract this from the mass number. # n = a – # p = 127 – 53 = 74. Copper is the 29th element of the periodic table so its atomic number is 29. the atomic number of an element is equal to the number of protons and electrons in that element. therefore, a copper atom has twenty nine protons and twenty nine electrons. the number of neutrons in an atom can be determined by the difference between the atomic mass. Number of neutrons in copper. the number of neutrons can be found by subtracting the atomic number from its atomic mass. number of neutrons in copper = atomic mass of copper – atomic number of copper = 64 – 29 = 35. number of electrons in copper. for a neutral atom, the number of electrons can be found by knowing the atomic number of that atom. Video: cu, cu , and cu2 electron configuration notation. in writing the electron configuration for copper the first two electrons will go in the 1s orbital. since 1s can only hold two electrons the next 2 electrons for copper go in the 2s orbital. the next six electrons will go in the 2p orbital. the p orbital can hold up to six electrons.

Comments are closed.