Heating Curve And Cooling Curve Of Water Enthalpy Of Fusion Vaporization

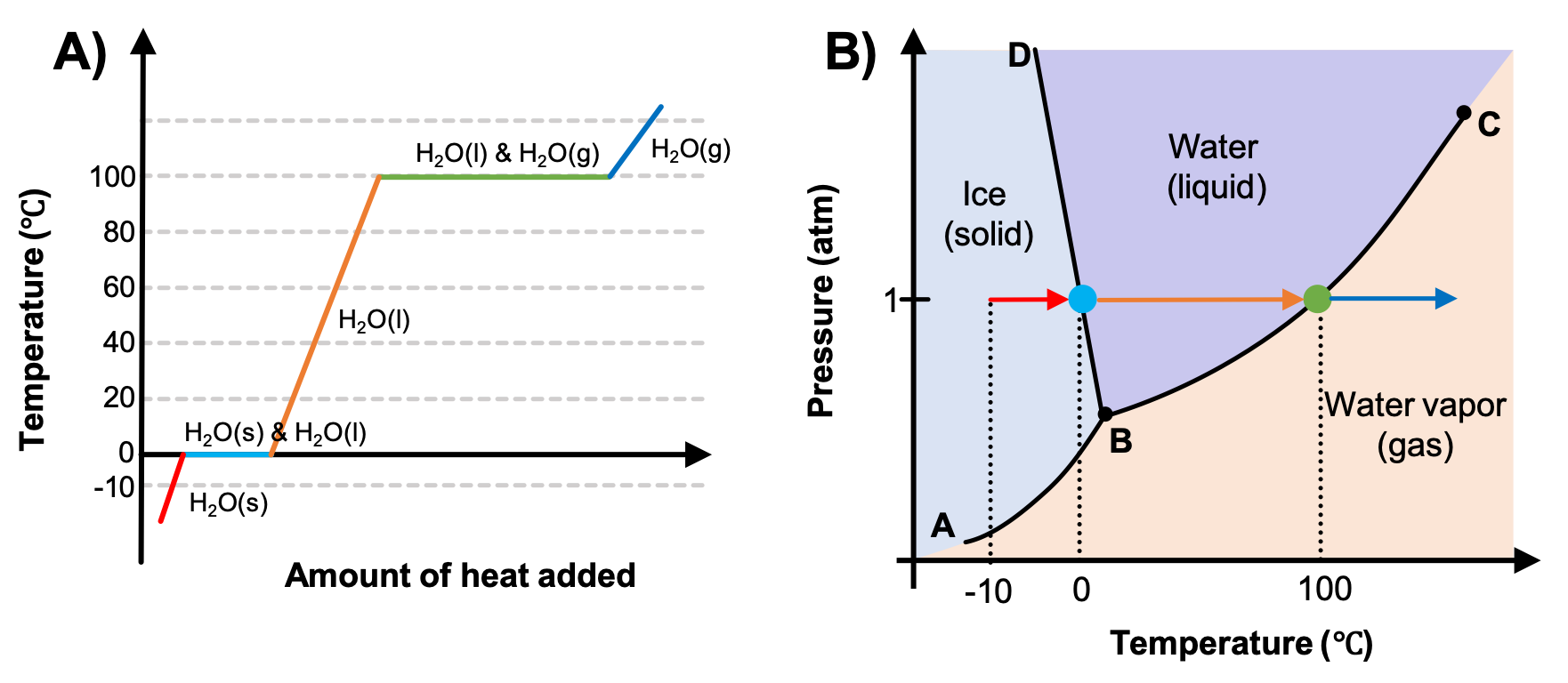
Heating Curve And Cooling Curve Of Water Enthalpy Of Fusionођ This chemistry video tutorial provides a basic introduction into the heating curve of water and the cooling curve of water. as heat is added to water, the t. Heating curves. figure 11.7.3 11.7. 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the sample is initially ice at 1 atm and −23°c; as heat is added, the temperature of the ice increases linearly with time. the slope of the line depends on both the mass of the ice and the specific heat (cs) of.

Heating Curve Of Water Enthalpy Of Fusion And Vaporization Youtu Heating curve and cooling curve of water enthalpy of fusion & vaporization. The energy change associated with each common phase change is shown in figure 2.5.1 2.5. 1. Δ h is positive for any transition from a more ordered to a less ordered state and negative for a transition from a less ordered to a more ordered state. previously, we defined the enthalpy changes associated with various chemical and physical processes. For many years drinking water has been cooled in hot cli mates by evaporating it from the surfaces of canvas bags or porous clay pots. how many grams of water can be cooled from 35 to 20 °c by the evaporation of 60 g of water? (the heat of vaporization of water in this temperature range is 2.4 kj g. the specific heat of water is 4.18 j g k). The equation for determining the enthalpy of fusion (Δh Δ h) is listed below. Δh = nΔhfus (1) (1) Δ h = n Δ h f u s. with. n n = number of moles. Δhfus Δ h f u s the molar heat of the substance. example 1 1. calculate the heat when 36.0 grams of water at 113 °c is cooled to 0 °c. given.
Heat Of Vaporization And Fusion For many years drinking water has been cooled in hot cli mates by evaporating it from the surfaces of canvas bags or porous clay pots. how many grams of water can be cooled from 35 to 20 °c by the evaporation of 60 g of water? (the heat of vaporization of water in this temperature range is 2.4 kj g. the specific heat of water is 4.18 j g k). The equation for determining the enthalpy of fusion (Δh Δ h) is listed below. Δh = nΔhfus (1) (1) Δ h = n Δ h f u s. with. n n = number of moles. Δhfus Δ h f u s the molar heat of the substance. example 1 1. calculate the heat when 36.0 grams of water at 113 °c is cooled to 0 °c. given. Heating curves. figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the sample is initially ice at 1 atm and −23°c; as heat is added, the temperature of the ice increases linearly with time. the slope of the line depends on both the mass of the ice and the specific heat (c s) of ice. Figure 10.28 for a given substance, the sum of its enthalpy of fusion and enthalpy of vaporization is approximately equal to its enthalpy of sublimation. heating and cooling curves in the chapter on thermochemistry, the relation between the amount of heat absorbed or released by a substance, q , and its accompanying temperature change, Δ t.

Heating And Cooling Curves Explained Heating curves. figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the sample is initially ice at 1 atm and −23°c; as heat is added, the temperature of the ice increases linearly with time. the slope of the line depends on both the mass of the ice and the specific heat (c s) of ice. Figure 10.28 for a given substance, the sum of its enthalpy of fusion and enthalpy of vaporization is approximately equal to its enthalpy of sublimation. heating and cooling curves in the chapter on thermochemistry, the relation between the amount of heat absorbed or released by a substance, q , and its accompanying temperature change, Δ t.
Heating And Cooling Curve Explanation

Comments are closed.