Heating And Cooling Curve Introduction Plus Kinetic A Vrogue Co
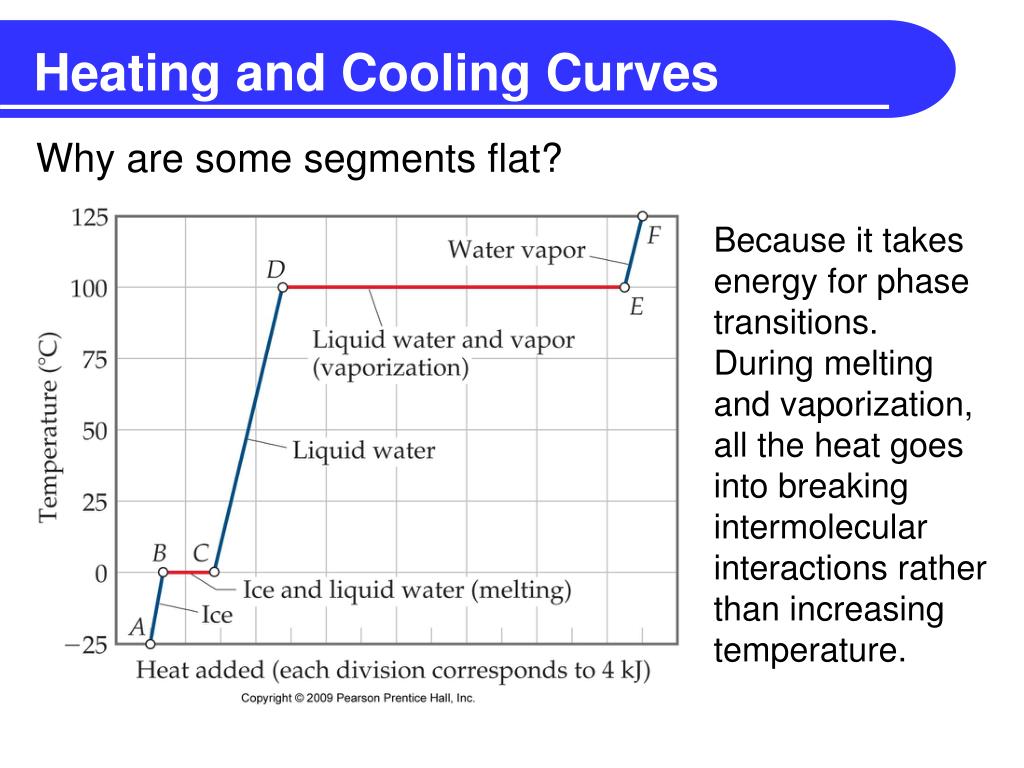
Heating And Cooling Curve Introduction Plus Kinetic A Vrogue Co An introduction to heating and cooling curve. in this video, i introduce heating and cooling curves and show the location of phase changes. " a typical heat. Preview. pchem mod 5. 76 terms. cloudydream77. preview. study with quizlet and memorize flashcards containing terms like heating curve, cooling curve, the solid phase kinetic energy is increasing potential energy is constant and more.

Heating And Cooling Curve Introduction Plus Kinetic A Vrogue Co Specific heat of steam. 1, 3, 5. which part of the graph is kinetic energy increasing? 2, 4. which part of the graph is potential energy increasing? t4. the sample starts as a gas, and heat is removed at constant rate. at which time does the sample contain the most liquid? study with quizlet and memorize flashcards containing terms like heat of. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of co 2 . the entire experiment could be run in reverse. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. the water could then be cooled to 0°c, at which point continued cooling would. Figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant. the change of state behavior of all substances can be represented with a heating curve of this type. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at 2861°c. cooling curves. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. just like heating curves, cooling curves have.
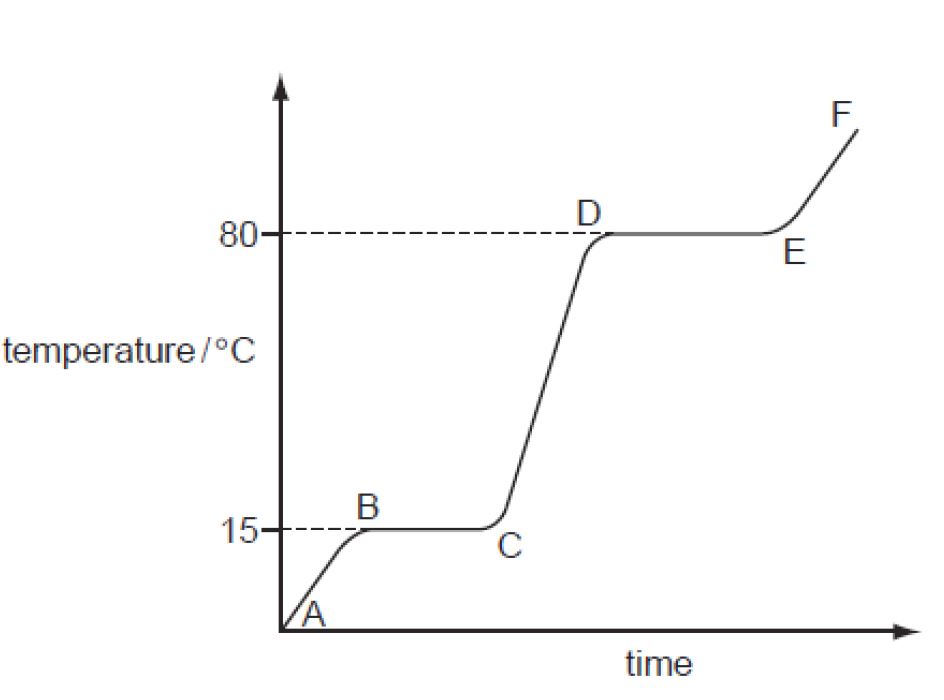
Heating And Cooling Curve Introduction Plus Kinetic A Vrogue Co Figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant. the change of state behavior of all substances can be represented with a heating curve of this type. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at 2861°c. cooling curves. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. just like heating curves, cooling curves have. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at. 2861°c. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. A quick note about cooling curves. let's say we wanted to go from steam to ice. we would use a cooling curve. the cooling curve is a mirror image of the heating curve. so, it will start at a high temperature and have downward diagonals. the diagonals alternate with plateaus. the flat lines are the enthalpy of condensation and freezing. remember.

Heating And Cooling Curve Introduction Plus Kinetic And Potentia For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at. 2861°c. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. A quick note about cooling curves. let's say we wanted to go from steam to ice. we would use a cooling curve. the cooling curve is a mirror image of the heating curve. so, it will start at a high temperature and have downward diagonals. the diagonals alternate with plateaus. the flat lines are the enthalpy of condensation and freezing. remember.

Comments are closed.