Heating And Cooling Curve Diagram

Heating And Cooling Curves Ck 12 Foundation The experiment described above can be summarized in a graph called a heating curve (figure below). figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant. Also described was the use of heating and cooling curves to determine a substance’s melting (or freezing) point. making such measurements over a wide range of pressures yields data that may be presented graphically as a phase diagram.

Heating And Cooling Curves Learn how to draw and interpret heating and cooling curves for substances that undergo changes of state. see examples of ice, water, and steam, and how they relate to melting, boiling, and sublimation points. Learn how to draw and interpret heating and cooling curves, which show the phase changes of a substance when heat is added or removed at a constant rate. see examples of heating ice to steam and cooling steam to ice, and how to calculate the heat energy involved. This chemistry video tutorial provides a basic introduction into the heating curve of water and the cooling curve of water. as heat is added to water, the t. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at 2861°c. cooling curves. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. just like heating curves, cooling curves have.
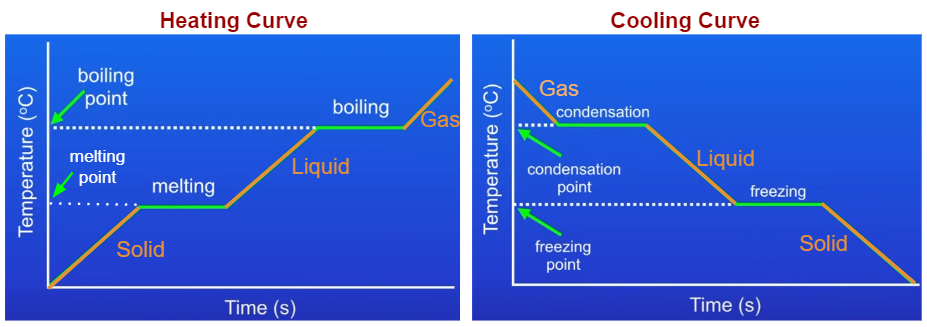
Heating And Cooling Graphs Examples Solutions Videos Notes This chemistry video tutorial provides a basic introduction into the heating curve of water and the cooling curve of water. as heat is added to water, the t. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at 2861°c. cooling curves. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. just like heating curves, cooling curves have. Understanding heating and cooling curves is crucial for grasping how substances absorb or release heat during phase changes. as a substance heats up, it undergoes an endothermic process, indicated by a positive heat variable (q), absorbing energy to break molecular bonds and transition from solid to liquid (melting or fusion) and eventually to gas (vaporization). Ethyl chloride (c2h5cl) boils at 12 °c. when liquid c2h5cl under pressure is sprayed on a room temperature (25 °c) surface in air, the surface is cooled considerably. (b) assume that the heat lost by the surface is gained by ethyl chloride.

Comments are closed.