Graph Between Stopping Potential Vs Frequency Explained By Er An
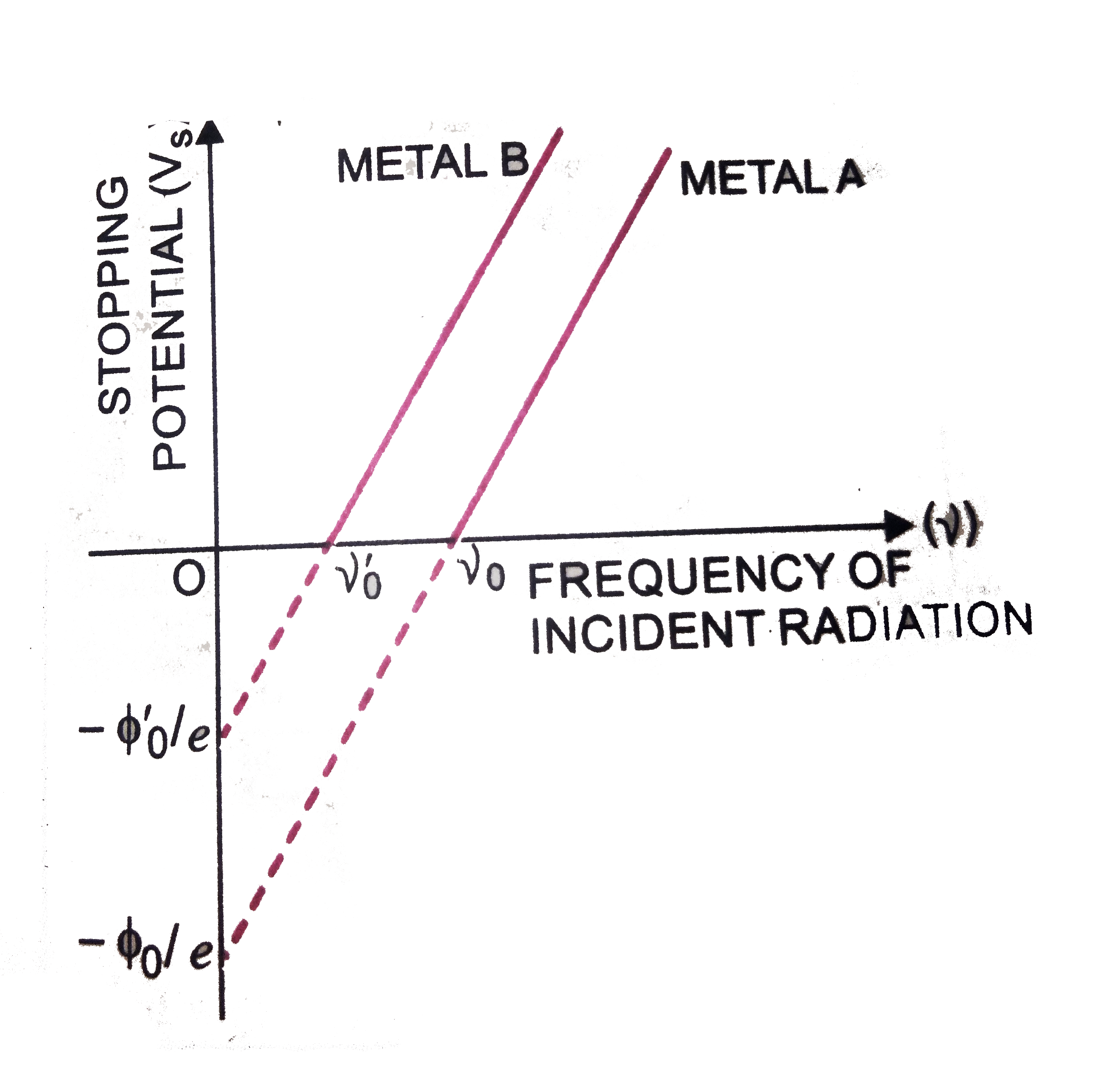
Sketch The Graphs Showing The Variation Of Stopping Potential Vs With Let's explore how the graph of stopping potential vs frequency can be used to calculate the planck's constant experimentally! from einstein's photoelectric e. Evstop =hf −e0 (4) according to (4), the stopping potential is a linearly increasing function of f: ( f f0) e h vstop = − (5) a graph of v stop vs. f would provide a way to measure planck’s constant h and the cutoff (or threshold) frequency f 0. apparatus notes the light source used in our apparatus consists of several interchangeable.
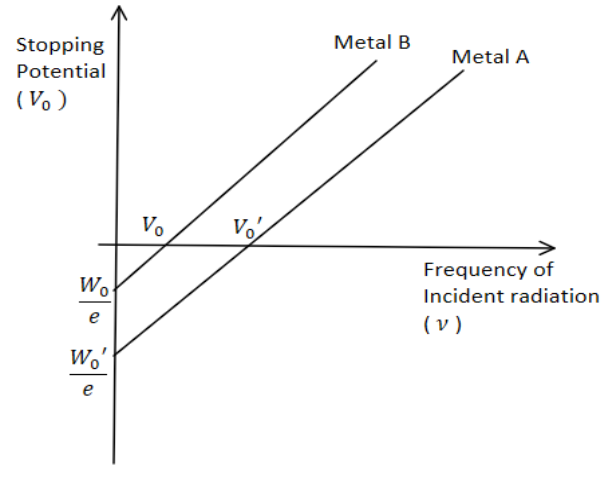
The Graph Shows Variation Of Stopping Potential V 0 Versus Lesson 34: photoelectric effect graphs like many other topics in science, the results of the photoelectric effect can be better understood if the results are presented in a graph. it is important to be able to interpret these graphs correctly. the following graph is a typical example of how the photoelectric effect will be shown to you. Figure 6.3.1 6.3. 1: an experimental setup to study the photoelectric effect. the anode and cathode are enclosed in an evacuated glass tube. the voltmeter measures the electric potential difference between the electrodes, and the ammeter measures the photocurrent. the incident radiation is monochromatic. On a graph of maximum kinetic energy vs. frequency… all curves are linear with slope equal to planck's constant. h = 6.63 × 10 −34 j s. the intercept on the energy axis is the threshold frequency of the material; magnify. classical physics cannot explain why… no photoelectrons are emitted when the incident light has a frequency below the. E k = ev s. now, we know that hf = φ e k (max) so we can now write: hf = φ evs. rearranging this we get: evs = hf φ. this has the form of a straight line graph. therefore if we perform an experiment, varying the frequency of the light we use to illuminate the metal plate and measuring the corresponding stopping potential we can present.
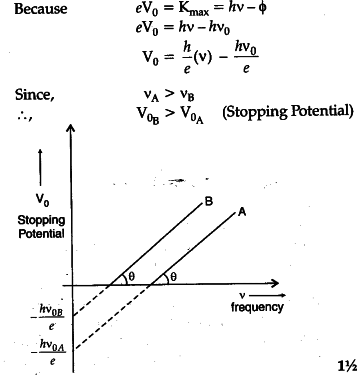
Sketch The Graphs Showing Variation Of Stopping Potential With On a graph of maximum kinetic energy vs. frequency… all curves are linear with slope equal to planck's constant. h = 6.63 × 10 −34 j s. the intercept on the energy axis is the threshold frequency of the material; magnify. classical physics cannot explain why… no photoelectrons are emitted when the incident light has a frequency below the. E k = ev s. now, we know that hf = φ e k (max) so we can now write: hf = φ evs. rearranging this we get: evs = hf φ. this has the form of a straight line graph. therefore if we perform an experiment, varying the frequency of the light we use to illuminate the metal plate and measuring the corresponding stopping potential we can present. Graph connecting 'ke max ' and frequency: maximum kinetic energy of photoelectrons versus frequency of incident radiation graph . now, if we increase the reverse potential, the photocurrent gradually decreases and becomes zero at a particular reverse potential. this minimum applied reverse potential is called stopping potential v 0. hence the. E = hf, (1) (1) e = h f, where e e is the energy of the radiation, f f is its frequency, and h h is planck's constant (6.63×10 34 js). the notion of light quantization was first introduced by planck. its validity is based on solid experimental evidence, most notably the photoelectric effect. the basic physical process underlying this effect.

Stopping Potential Vs Frequency Scatter Chart Made By Kgmaxson Plotly Graph connecting 'ke max ' and frequency: maximum kinetic energy of photoelectrons versus frequency of incident radiation graph . now, if we increase the reverse potential, the photocurrent gradually decreases and becomes zero at a particular reverse potential. this minimum applied reverse potential is called stopping potential v 0. hence the. E = hf, (1) (1) e = h f, where e e is the energy of the radiation, f f is its frequency, and h h is planck's constant (6.63×10 34 js). the notion of light quantization was first introduced by planck. its validity is based on solid experimental evidence, most notably the photoelectric effect. the basic physical process underlying this effect.

Comments are closed.