Draw Additional Resonance Structures For Each Ion
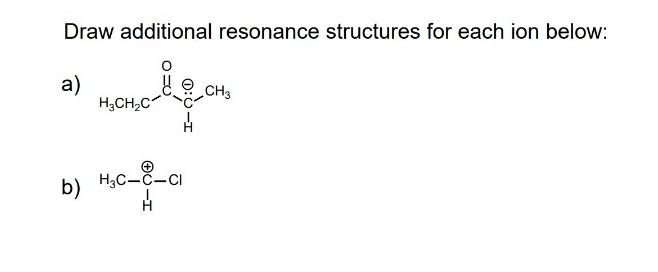
Solved Draw Additional Resonance Structures For Each Ion Chegg Exercise 2.5.1 2.5. 1. draw the resonance contributors that correspond to the curved, two electron movement arrows in the resonance expressions below. then identify the type of resonance motion in each structure below. answer. Exercise 2.4.5 2.4. 5. below is a minor resonance contributor of a species known as an ‘enamine’, which we will study more in section 19.8 (formation of enamines) section 23.12 (reactions of enamines). draw the major resonance contributor for the enamine, and explain why your contributor is the major one. answer.
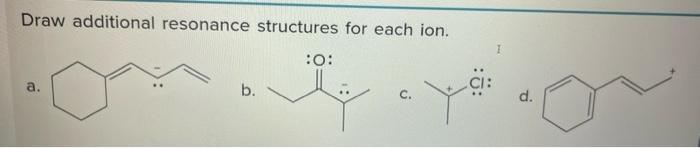
Solved Draw Additional Resonance Structures For Each Ion 1 Chegg Example 2.6.3: carbonate ion. consider the carbonate ion: co 32 . solution. step 1: draw the lewis structure & resonance. step 2: combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed. step 3: add only the lone pairs found on all resonance structures. 2.6 • drawing resonance forms look back at the resonance forms of the acetate ion and the acetone anion shown in the previous section. the pattern seen there is a common one that leads to a useful technique for drawing resonance forms. in general, any three atom grouping with a p orbital on each atom has two resonance forms:. Example 1: co32 ion. step 1. calculate the total number of valence electrons from each atom. carbon atom = 4. oxygen atoms (3*6) = 18. for ( 2) charge = 2. ** consider the 2 charge at the last step (i.e. this molecule has two extra electrons). step 2. when there is more than one type of atom, keep the least electronegative or metallic atom as. Guidelines to follow. to draw all resonance structures, take the lewis structure we drawn by using vespr rule. in resonance structures, it does not require to show transformation of electrons by arrows. but, to identify each resonance structures, it is good to show arrows. in following examples, arrows are used to show electrons transformation.

Solved Draw Additional Resonance Structures For Each Ion 1 Chegg Example 1: co32 ion. step 1. calculate the total number of valence electrons from each atom. carbon atom = 4. oxygen atoms (3*6) = 18. for ( 2) charge = 2. ** consider the 2 charge at the last step (i.e. this molecule has two extra electrons). step 2. when there is more than one type of atom, keep the least electronegative or metallic atom as. Guidelines to follow. to draw all resonance structures, take the lewis structure we drawn by using vespr rule. in resonance structures, it does not require to show transformation of electrons by arrows. but, to identify each resonance structures, it is good to show arrows. in following examples, arrows are used to show electrons transformation. Resonance structures examples [2,4,7]. 1. ozone (o 3). ozone has two major resonance structures that contribute equally to its overall hybrid structure. each oxygen atom has 6 valence electrons, making it a total of 18 for the molecule. 6 out of 18 electrons participate in chemical bonds, and the remaining 12 remain as lone pairs. Calculating formal charge from lewis structures assign formal charges to each atom in the interhalogen ion icl 4 −. icl 4 −. solution. step 1. we divide the bonding electron pairs equally for all i–cl bonds: step 2. we assign lone pairs of electrons to their atoms. each cl atom now has seven electrons assigned to it, and the i atom has.
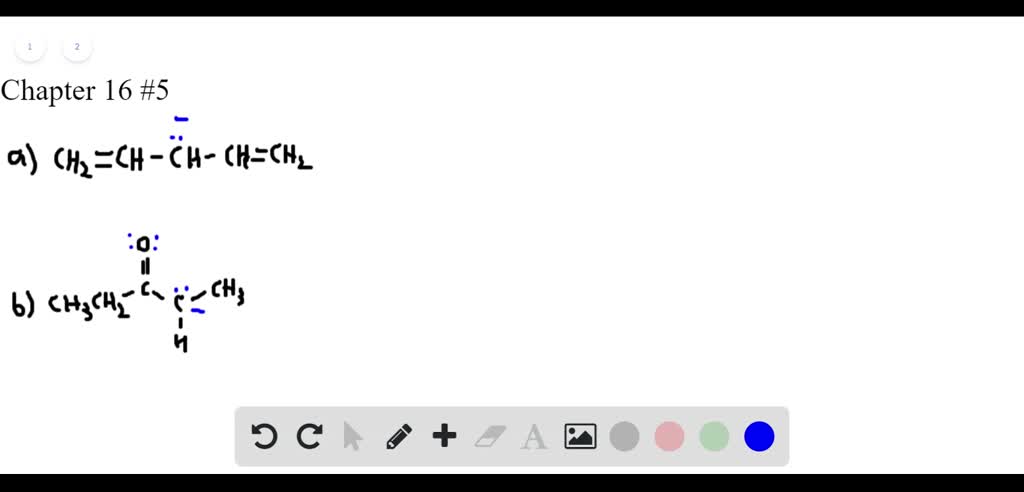
Solved Draw Additional Resonance Structures For Each Ion Resonance structures examples [2,4,7]. 1. ozone (o 3). ozone has two major resonance structures that contribute equally to its overall hybrid structure. each oxygen atom has 6 valence electrons, making it a total of 18 for the molecule. 6 out of 18 electrons participate in chemical bonds, and the remaining 12 remain as lone pairs. Calculating formal charge from lewis structures assign formal charges to each atom in the interhalogen ion icl 4 −. icl 4 −. solution. step 1. we divide the bonding electron pairs equally for all i–cl bonds: step 2. we assign lone pairs of electrons to their atoms. each cl atom now has seven electrons assigned to it, and the i atom has.

Comments are closed.