Determining The Empirical Formula Of Magnesium Oxide Experiment

Empirical Formula Of Magnesium Oxide Postlab Analysis Youtube Experiment 602: empirical formula . section 1: purpose and summary . determine the empirical formula of magnesium oxide. calculate the mass of oxygen using weighing by difference. calculate the mole of a sample from its mass. in this experiment, students will conduct the reaction between magnesium and oxygen gas. The empirical formula is the simplest whole number ratio of atoms in a compound. the ratio of atoms is the same as the ratio of moles. so our job is to calculate the molar ratio of mg to o. mass of mg = 0.297 g. mass of magnesium oxide = mass of mg mass of o. 0.493 g = 0.297 g mass of o. mass of o = (0.493 – 0.297) g = 0.196 g.

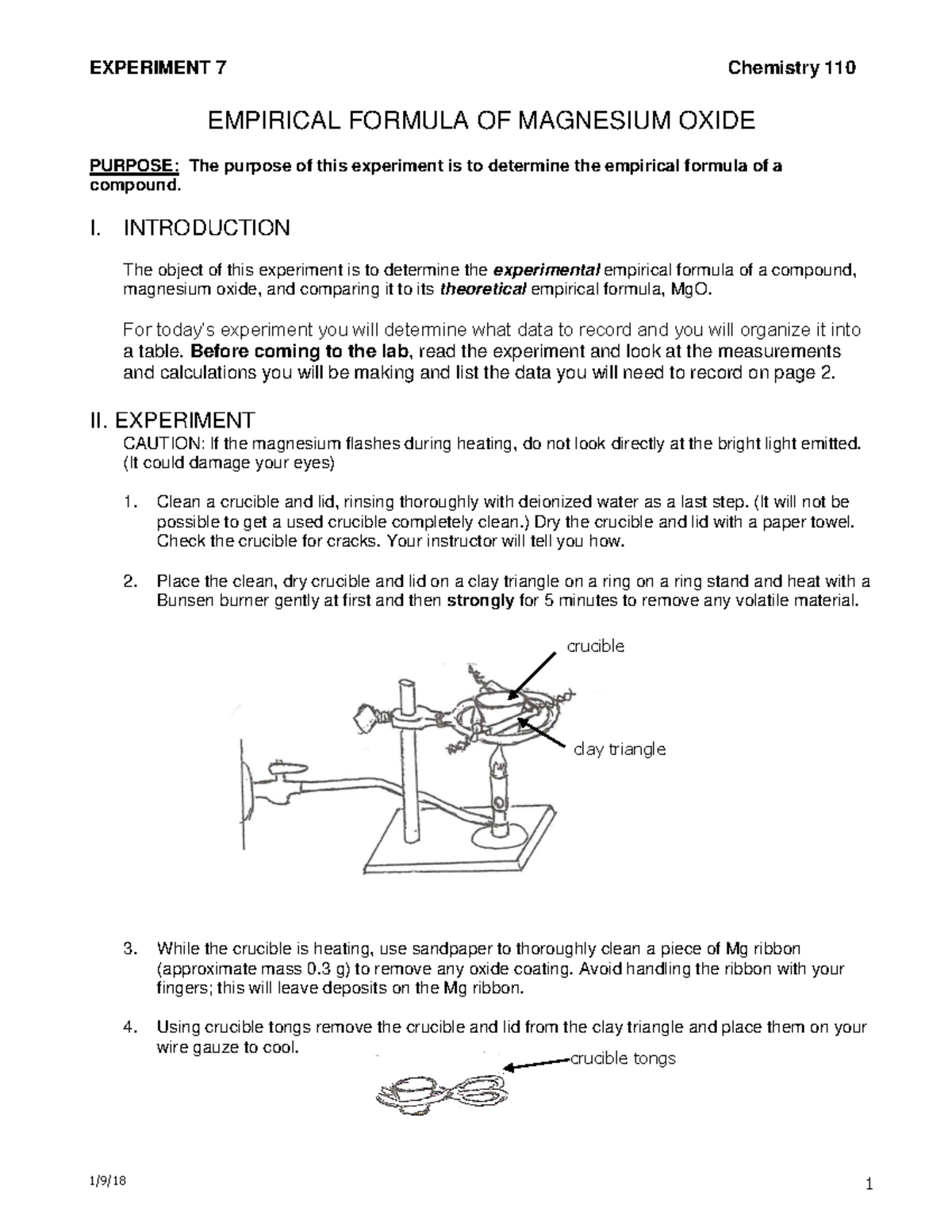
Determining The Empirical Formula Of Magnesium Oxide Lab The object of this experiment is to determine the experimental empirical formula of a compound, magnesium oxide, and comparing it to its theoretical empirical formula, mgo. a table. before coming to the lab, read the experiment and look at the measurements and calculations you will be making and list the data you will need to record on page 2. ii. Experiment to determining the empirical formula of magnesium oxide. in this experiment we will be using a piece of apparatus known as a crucible. a crucible is ceramic vessel with a lid used to hold substances that are heated to high temperatures. a crucible is preferable to using a glass test tube in this experiment because:. To determine the empirical formula of magnesium oxide. mass of crucible and lid (step 1) 50.00 mass of crucible, lid and magnesium (step 3) 50.24 mass of crucible, lid and magnesium oxide (step 7. The goal of this experiment is to determine the empirical formula of magnesium oxide experimentally. objectives. 1. to burn a sample of magnesium in air and measure the gain in mass. 2. to determine the empirical formula of magnesium oxide. 3. to find the theoretical and experimental yields of magnesium oxide, and report statistics on the.
Magnesium Oxide Lab To determine the empirical formula of magnesium oxide. mass of crucible and lid (step 1) 50.00 mass of crucible, lid and magnesium (step 3) 50.24 mass of crucible, lid and magnesium oxide (step 7. The goal of this experiment is to determine the empirical formula of magnesium oxide experimentally. objectives. 1. to burn a sample of magnesium in air and measure the gain in mass. 2. to determine the empirical formula of magnesium oxide. 3. to find the theoretical and experimental yields of magnesium oxide, and report statistics on the. Lab 2 – determination of the empirical formula of magnesium oxide. goal and overview. the quantitative stoichiometric relationships governing mass and amount will be studied using the combustion reaction of magnesium metal. magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such as h2o for water or ho for hydrogen peroxide). when magnesium and oxygen are heated together, they react: magnesium oxygen magnesium oxide rxn 1 from the masses of magnesium and oxygen that combine, we can calculate the.

Determining The Empirical Formula Of Magnesium Oxide Lab Course Hero Lab 2 – determination of the empirical formula of magnesium oxide. goal and overview. the quantitative stoichiometric relationships governing mass and amount will be studied using the combustion reaction of magnesium metal. magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. The empirical formula of a compound merely gives the ratio of atoms in the compound based on experimental evidence (such as h2o for water or ho for hydrogen peroxide). when magnesium and oxygen are heated together, they react: magnesium oxygen magnesium oxide rxn 1 from the masses of magnesium and oxygen that combine, we can calculate the.

Empirical Formula Of Magnesium Oxide Calculation Youtube

Comments are closed.