Covid 19 Rapid Test Safecare For Antigens Influenza A Influen
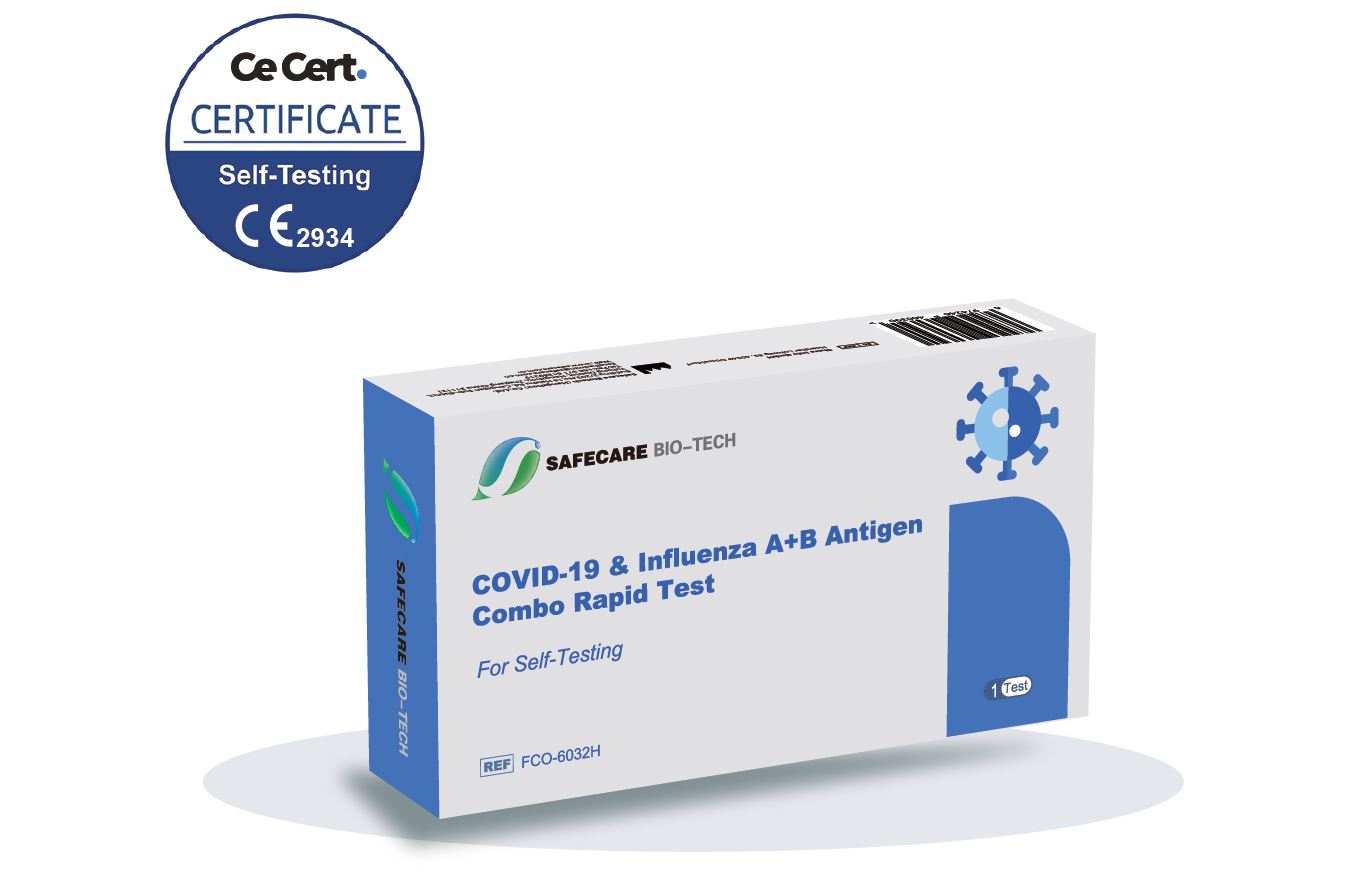
Safecare Influenza A B Antigen Covid 19 Combo Rapid Test 10222 Your sample(s) was tested for covid 19 and influenza using the ihealth covid 19 flu a&b rapid test pro. this fact sheet contains information to help you understand the risks and benefits of using. Storage and stability. store the ihealth covid 19 flu a&b rapid test pro at room temperature 36 86°f (2 30°c) in the original packaging, away from direct sunlight. ensure all test components are.

Covid 19 Rapid Test Safecare For Antigens Influenza A Influen Wash your hands with soap and water for 20 seconds and dry them thoroughly, or use hand sanitizer. locate the tube holder on the box (look for the red circle on the kit's box). insert the buffer. The speedy swab rapid covid 19 flu a&b antigen self test is a lateral flow immunoassay intended for the qualitative detection and differentiation of sars cov 2, influenza a, and influenza b protein antigens. this test is authorized for non prescription home use with self collected anterior nasal swab specimens from individuals aged 14 years. Feel safe wherever you go with our covid 19 flu a&b 3 in 1 test kit. its compact design ensures not only easy storage but also convenient portability in your travel bag, allowing you to seamlessly take charge of your health during travels, outings, or any time you need assurance. stay vigilant and protected, especially when flu covid cases have. But the test in question detects the markers of three different types of virus—sars cov 2 (which causes covid 19) influenza a and influenza b. images of the rapid test in question show it is a combination dual test, with two panels—one detects covid 19 antigens and another that detects influenza a and b. depending on which lines appear, the.
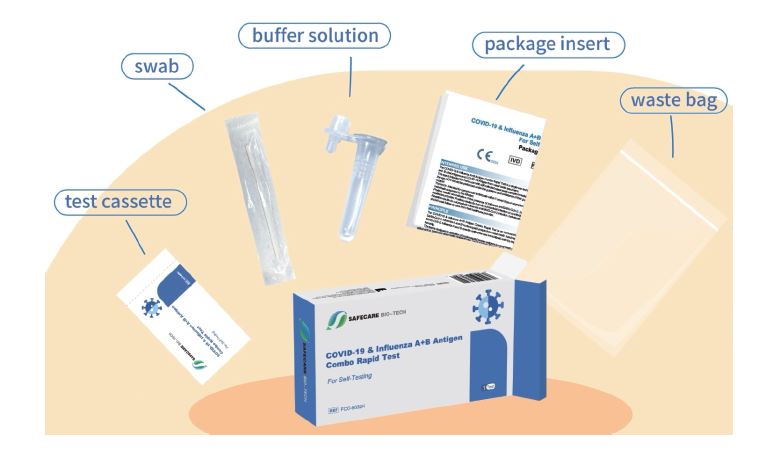
Safecare Influenza A B Antigen Covid 19 Combo Rapid Test 10222 Feel safe wherever you go with our covid 19 flu a&b 3 in 1 test kit. its compact design ensures not only easy storage but also convenient portability in your travel bag, allowing you to seamlessly take charge of your health during travels, outings, or any time you need assurance. stay vigilant and protected, especially when flu covid cases have. But the test in question detects the markers of three different types of virus—sars cov 2 (which causes covid 19) influenza a and influenza b. images of the rapid test in question show it is a combination dual test, with two panels—one detects covid 19 antigens and another that detects influenza a and b. depending on which lines appear, the. If the second antigen test is negative, per fda guidance, a third antigen test could be considered if there is a high clinical suspicion of covid 19; influenza testing test for influenza if results will change clinical management or for infection control decisions (e.g. long term care facility resident returning to a facility, or a person of. Kit is an aid in the differentiate of patients with suspected sars cov 2 influenza human nasal swab samples from individuals within seven days of symptom onset. a influenza for covid 19 b infection and within in four conjunction days of symptom onset for influenza a b. with clinical symptoms and result of other diagnostic this test is used.
Covid 19 Rapid Test Safecare For Antigens Influenza A Influen If the second antigen test is negative, per fda guidance, a third antigen test could be considered if there is a high clinical suspicion of covid 19; influenza testing test for influenza if results will change clinical management or for infection control decisions (e.g. long term care facility resident returning to a facility, or a person of. Kit is an aid in the differentiate of patients with suspected sars cov 2 influenza human nasal swab samples from individuals within seven days of symptom onset. a influenza for covid 19 b infection and within in four conjunction days of symptom onset for influenza a b. with clinical symptoms and result of other diagnostic this test is used.

Comments are closed.