Copper Periodic Table And Atomic Properties

Copper Uses Properties Facts Britannica Biological role. copper is an essential element. an adult human needs around 1.2 milligrams of copper a day, to help enzymes transfer energy in cells. excess copper is toxic. genetic diseases, such as wilson’s disease and menkes’ disease, can affect the body’s ability to use copper properly. Copper is the 29th element in the periodic table and has a symbol of cu and atomic number of 29. it has an atomic weight of 63.546 and a mass number of 63. copper has twenty nine protons and thirty four neutrons in its nucleus, and twenty nine electrons in four shells. it is located in group eleven, period four and block d of the periodic table.
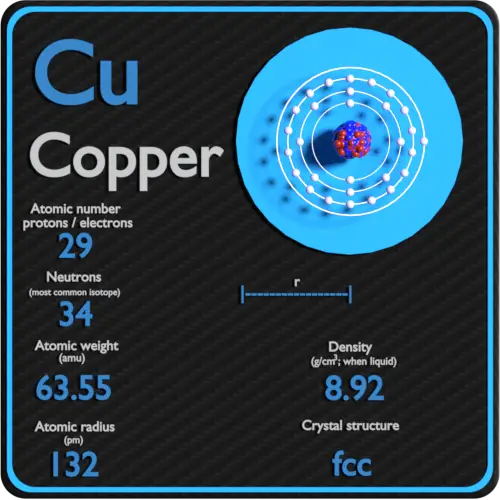
Copper Periodic Table And Atomic Properties Copper. face centered cubic (fcc) (cf4) copper is a chemical element; it has symbol cu (from latin cuprum) and atomic number 29. it is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. a freshly exposed surface of pure copper has a pinkish orange color. copper is used as a conductor of heat and electricity. Copper (cu), chemical element, a reddish, extremely ductile metal of group 11 (ib) of the periodic table that is an unusually good conductor of electricity and heat. copper is found in the free metallic state in nature. this native copper was first used (c. 8000 bce) as a substitute for stone by neolithic (new stone age) humans. Properties: copper has a melting point of 1083.4 0.2°c, boiling point of 2567°c, specific gravity of 8.96 (20°c), with a valence of 1 or 2. copper is reddish colored and takes a bright metallic luster. it is malleable, ductile, and a good conductor of electricity and heat. it is second only to silver as an electrical conductor. Atomic number of copper. copper is a chemical element with atomic number 29 which means there are 29 protons and 29 electrons in the atomic structure. the chemical symbol for copper is cu. the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. the nucleus is composed of protons and neutrons.
Copper Element Periodic Table Learnool Properties: copper has a melting point of 1083.4 0.2°c, boiling point of 2567°c, specific gravity of 8.96 (20°c), with a valence of 1 or 2. copper is reddish colored and takes a bright metallic luster. it is malleable, ductile, and a good conductor of electricity and heat. it is second only to silver as an electrical conductor. Atomic number of copper. copper is a chemical element with atomic number 29 which means there are 29 protons and 29 electrons in the atomic structure. the chemical symbol for copper is cu. the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. the nucleus is composed of protons and neutrons. Group name: coinage metal. period in periodic table: 4. block in periodic table: d. shell structure: 2.8.18.1. cas registry: 7440 50 8. copper atoms have 29 electrons and the shell structure is 2.8.18.1. the ground state electronic configuration of neutral copper is [ar]. 3d10. 4s1 and the term symbol of copper is 2s1 2. Copper properties, history, name origin, facts, applications, isotopes, electronic configuation, crystal structure, hazards and more; interactive periodic table of the chemical elements. copper periodic table.

Comments are closed.