Copper Atomic Number Atomic Mass Density Of Copper Nuclear
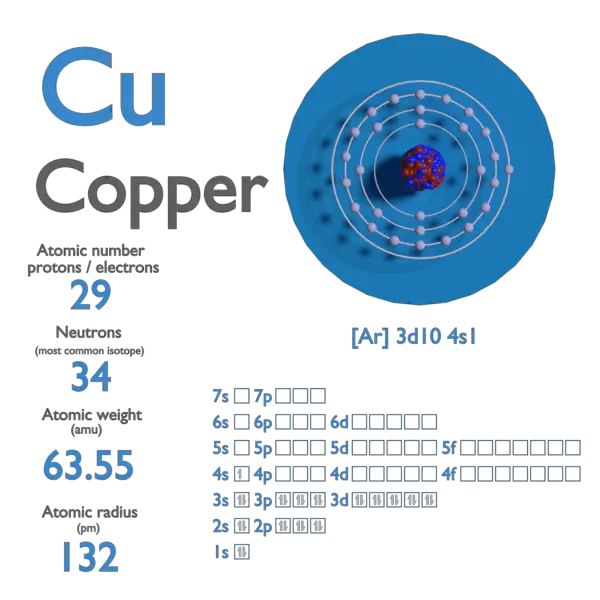
Copper Atomic Number Atomic Mass Density Of Copper Nuclear For 63 cu, the atomic mass is less than 63, so this must be the dominant factor. the atomic mass number determines especially the atomic mass of atoms. the mass number is different for each different isotope of a chemical element. how does the atomic mass determine the density of materials? density of copper. density of copper is 8.92g cm 3. Density (g cm −3) density is the mass of a substance that would fill 1 cm 3 at room temperature. relative atomic mass the mass of an atom relative to that of carbon 12. this is approximately the sum of the number of protons and neutrons in the nucleus. where more than one isotope exists, the value given is the abundance weighted average. isotopes.
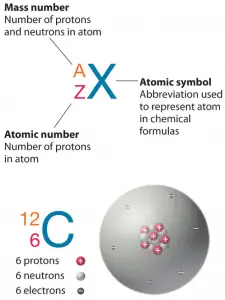
Copper Atomic Number Atomic Mass Density Of Copper Nuclear Copper is the 29th element in the periodic table and has a symbol of cu and atomic number of 29. it has an atomic weight of 63.546 and a mass number of 63. copper has twenty nine protons and thirty four neutrons in its nucleus, and twenty nine electrons in four shells. it is located in group eleven, period four and block d of the periodic table. Atomic and nuclear properties of copper (cu) quantity: units: value: units: atomic number: 29 : atomic mass: 63.546(3) g mole 1 x ray mass attenuation. The ratio of the average mass per atom of an isotope to 1 12 the mass of a carbon 12 atom. relative atomic mass is also known as atomic weight (symbol: a r). m: copper (cu) has an atomic mass of 29. find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Atomic mass of copper is 63.546 u. the atomic mass is the mass of an atom. the atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. the atomic mass is carried by the atomic nucleus, which occupies only about 10 12 of the total volume of the atom or.

Copper Atomic Number Atomic Mass Density Of Copper Nu Vrog The ratio of the average mass per atom of an isotope to 1 12 the mass of a carbon 12 atom. relative atomic mass is also known as atomic weight (symbol: a r). m: copper (cu) has an atomic mass of 29. find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Atomic mass of copper is 63.546 u. the atomic mass is the mass of an atom. the atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. the atomic mass is carried by the atomic nucleus, which occupies only about 10 12 of the total volume of the atom or. Mass numbers of typical isotopes of copper are 63; 65. atomic mass of copper. atomic mass of copper is 63.546 u. the atomic mass is the mass of an atom. the atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. the atomic mass is carried by the atomic. Copper. face centered cubic (fcc) (cf4) copper is a chemical element; it has symbol cu (from latin cuprum) and atomic number 29. it is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. a freshly exposed surface of pure copper has a pinkish orange color. copper is used as a conductor of heat and electricity.
Copper Atomic Number Atomic Mass Density Of Copper Nu Vrog Mass numbers of typical isotopes of copper are 63; 65. atomic mass of copper. atomic mass of copper is 63.546 u. the atomic mass is the mass of an atom. the atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. the atomic mass is carried by the atomic. Copper. face centered cubic (fcc) (cf4) copper is a chemical element; it has symbol cu (from latin cuprum) and atomic number 29. it is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. a freshly exposed surface of pure copper has a pinkish orange color. copper is used as a conductor of heat and electricity.

Comments are closed.