Cooling Curve Of Water Diagram
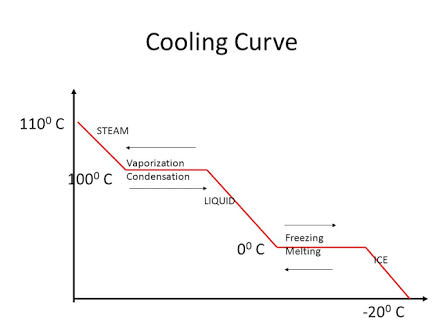
Draw The Labeled Graph For Cooling Curve Of Water At 100 C Bfdqi4nn Heating curves. figure 11.7.3 11.7. 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the sample is initially ice at 1 atm and −23°c; as heat is added, the temperature of the ice increases linearly with time. the slope of the line depends on both the mass of the ice and the specific heat (cs) of. This chemistry video tutorial provides a basic introduction into the heating curve of water and the cooling curve of water. as heat is added to water, the t.
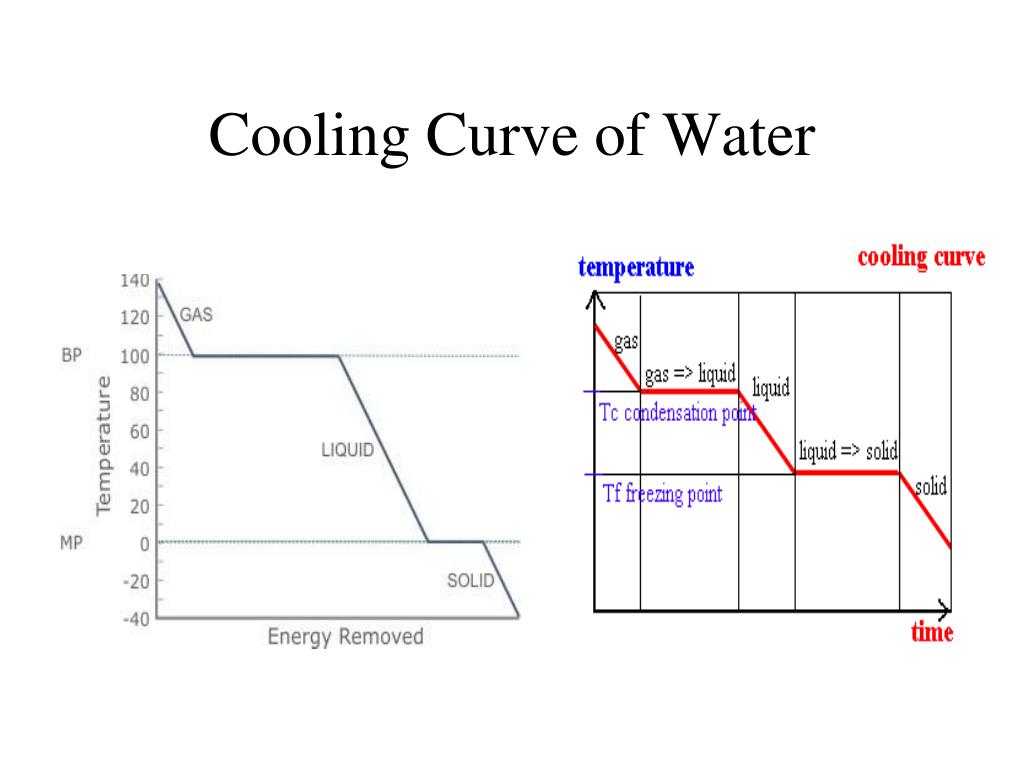
Ppt Heating And Cooling Curves Of Water Powerpoint Presentation Id A quick note about cooling curves. let's say we wanted to go from steam to ice. we would use a cooling curve. the cooling curve is a mirror image of the heating curve. so, it will start at a high temperature and have downward diagonals. the diagonals alternate with plateaus. the flat lines are the enthalpy of condensation and freezing. remember. The experiment described above can be summarized in a graph called a heating curve (figure below). figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of co 2 . the entire experiment could be run in reverse. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. the water could then be cooled to 0°c, at which point continued cooling would. Boil water. heat steam from 100 °c to 120 °c. the heat needed to change the temperature of a given substance (with no change in phase) is: q = m × c × Δ t (see previous chapter on thermochemistry). the heat needed to induce a given change in phase is given by q = n × Δ h. using these equations with the appropriate values for specific.

Heating Curve Of Water Explained The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of co 2 . the entire experiment could be run in reverse. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. the water could then be cooled to 0°c, at which point continued cooling would. Boil water. heat steam from 100 °c to 120 °c. the heat needed to change the temperature of a given substance (with no change in phase) is: q = m × c × Δ t (see previous chapter on thermochemistry). the heat needed to induce a given change in phase is given by q = n × Δ h. using these equations with the appropriate values for specific. By removing the time axis from the curves and replacing it with composition, the cooling curves indicate the temperatures of the solidus and liquidus for a given composition. this allows the solidus and liquidus to be plotted to produce the phase diagram: this page titled 12.5: interpretation of cooling curves is shared under a cc by nc sa. The water could then be cooled to 0°c, at which point continued cooling would freeze the water to ice. the ice could then be cooled to some point below 0°c. this could be diagrammed in a cooling curve that would be the reverse of the heating curve.
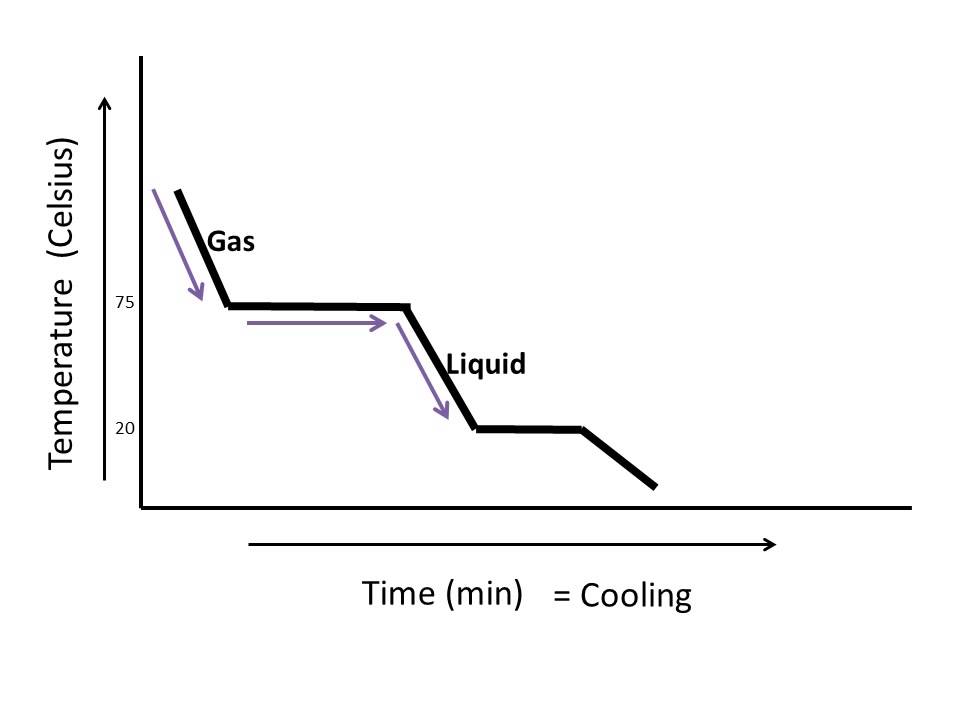
How To Read A Cooling Curve Youtube By removing the time axis from the curves and replacing it with composition, the cooling curves indicate the temperatures of the solidus and liquidus for a given composition. this allows the solidus and liquidus to be plotted to produce the phase diagram: this page titled 12.5: interpretation of cooling curves is shared under a cc by nc sa. The water could then be cooled to 0°c, at which point continued cooling would freeze the water to ice. the ice could then be cooled to some point below 0°c. this could be diagrammed in a cooling curve that would be the reverse of the heating curve.

Comments are closed.