Clinical Trial Phases With Review And Approval Process Clinical
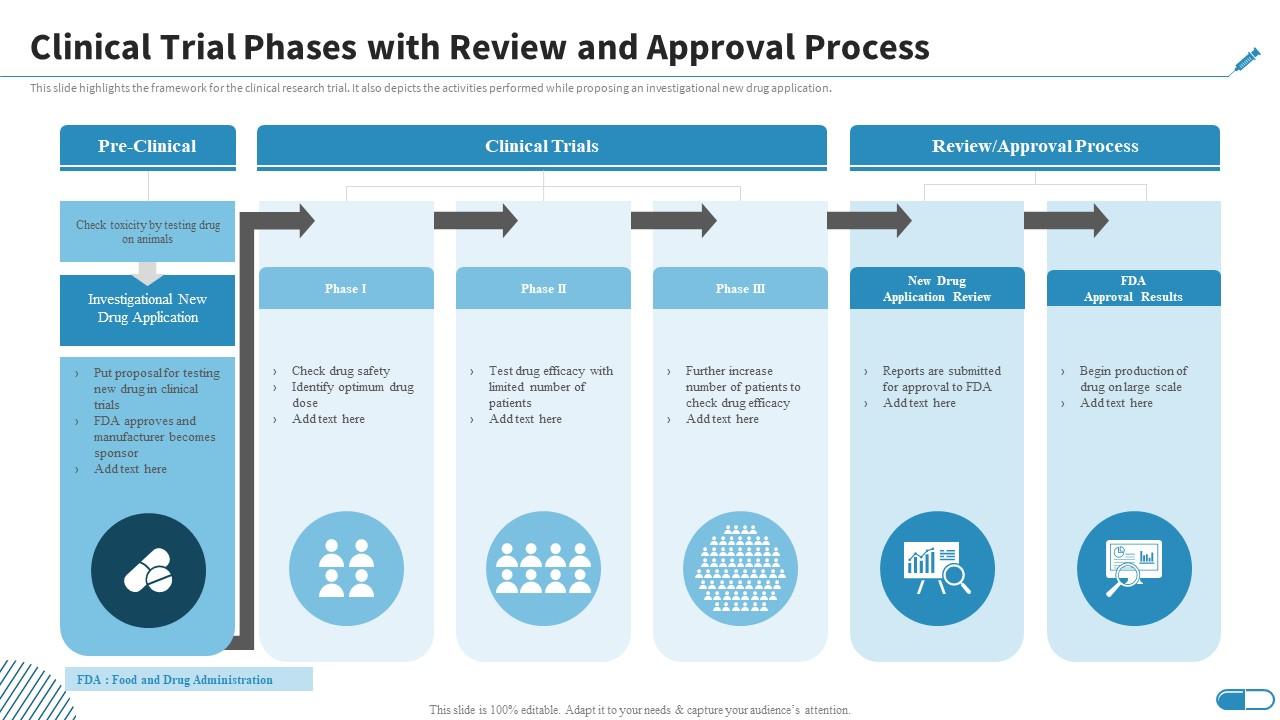
Clinical Trial Phases With Review And Approval Process Research The clinical trial process involves protocol development, designing a case record report form (crf), and functioning of institutional review boards (irbs). it also includes data management and the monitoring of clinical trial site activities. the crf is the most significant document in a clinical study. If the fda gives the green light, the investigational drug will enter several phases of clinical trials and post marketing approval: phase 1: phase 1 focuses on safety. about 20 to 80 healthy volunteers are recruited to establish a drug's safety and side effects and takes about 1 year. absorption, metabolism and excretion of the drug are also.
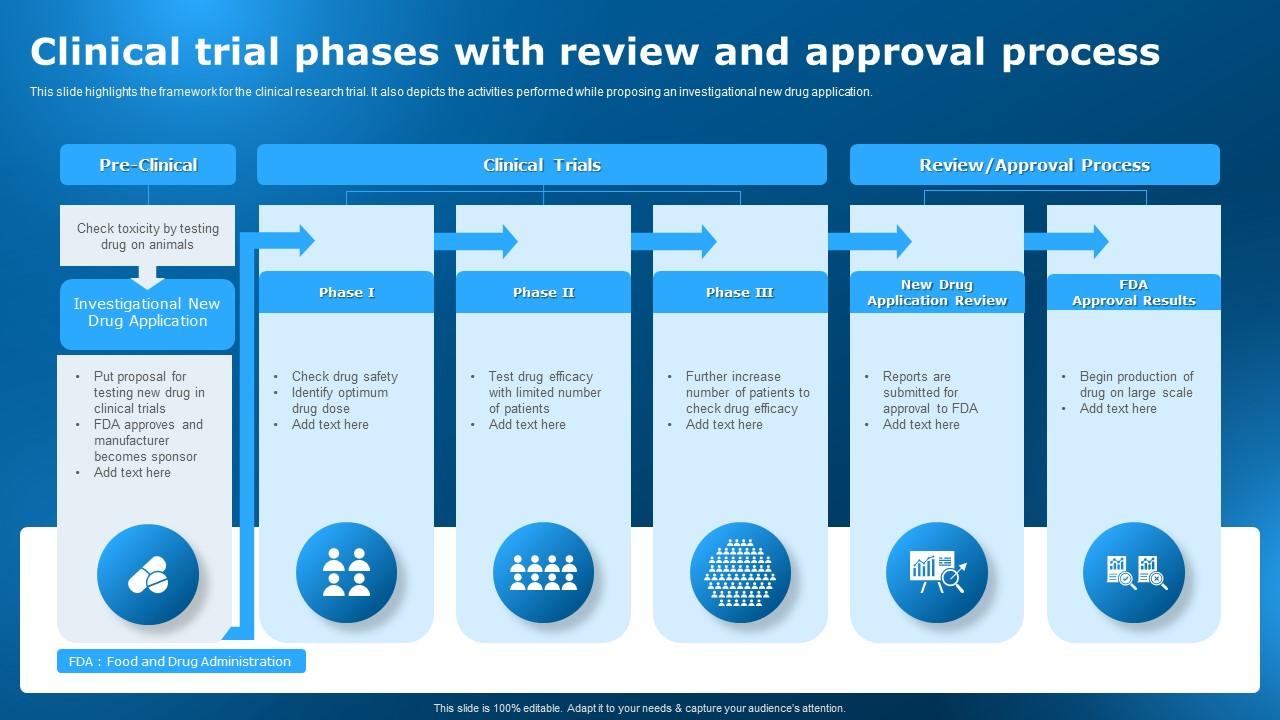
Clinical Trial Phases With Review And Approval Process Clinical The path to approval: clinical research phases. new treatments go through the following phases of clinical research: phase o trials. first in human clinical trial (optional) with 10 to 15 healthy volunteers where small amounts of the investigational drug are given to check if the drug behaves as expected in humans. The process protects volunteers who participate in clinical trials from unreasonable and significant risk in clinical trials. fda responds to ind applications in one of two ways: approval to begin. The ind stage consists of three phases. in phase i, clinical trials using healthy individuals are conducted to determine the drug’s basic properties and safety profile in humans. typically the drug remains in this stage for one to two years. in phase ii, efficacy trials begin as the drug is administered to volunteers of the target population. Clinical trial phases, the drug approval process, and measuring responses in science and medicine, information is constantly changing and may become out of date as new data emerge. all articles and interviews are informational only, should never be considered medical advice, and should never be acted on without review with your health care team.
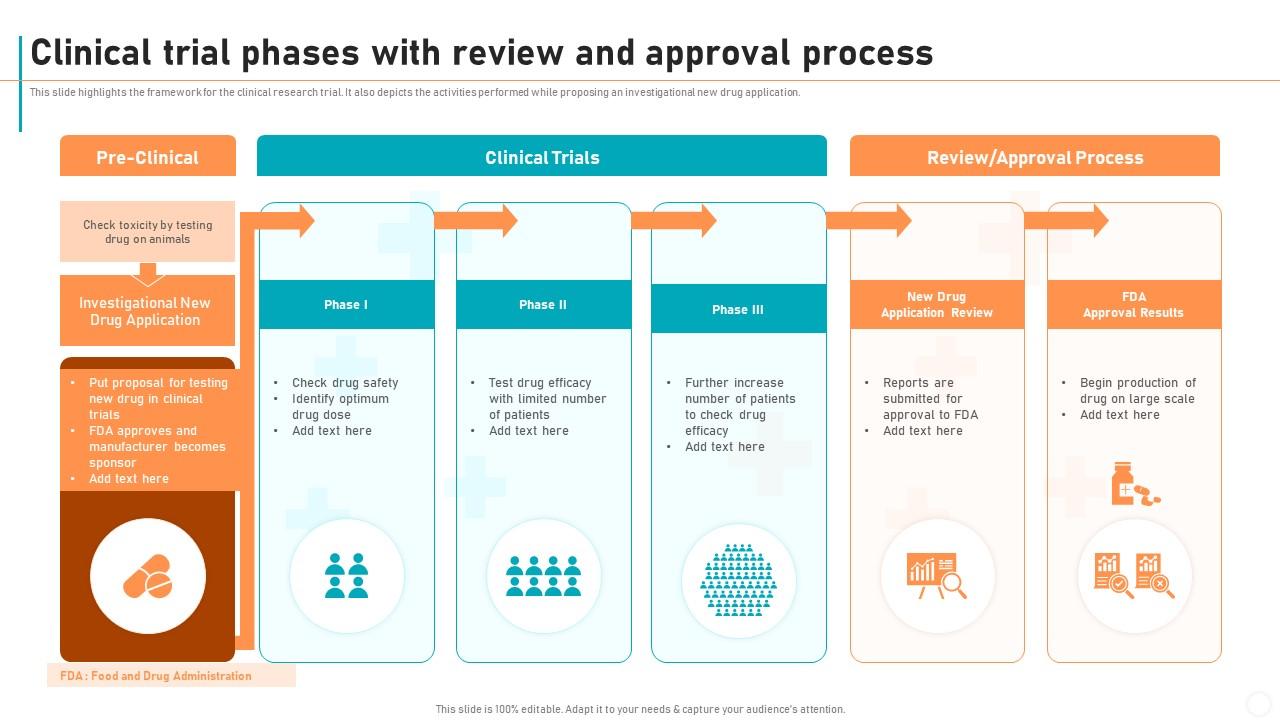
New Drug Development Process Clinical Trial Phases With Review And The ind stage consists of three phases. in phase i, clinical trials using healthy individuals are conducted to determine the drug’s basic properties and safety profile in humans. typically the drug remains in this stage for one to two years. in phase ii, efficacy trials begin as the drug is administered to volunteers of the target population. Clinical trial phases, the drug approval process, and measuring responses in science and medicine, information is constantly changing and may become out of date as new data emerge. all articles and interviews are informational only, should never be considered medical advice, and should never be acted on without review with your health care team. This narrative review is based on a course in clinical trials developed by one of the authors (djm), and is supplemented by a pubmed search predating january 2011 using the keywords “randomized controlled trial,” “patient clinical research,” “ethics,” “phase iv,” “data and safety monitoring board,” and “surrogate endpoint.”. Phase 0 of a clinical trial is done with a very small number of people, usually fewer than 15. investigators use a very small dose of medication to make sure it isn’t harmful to humans before.
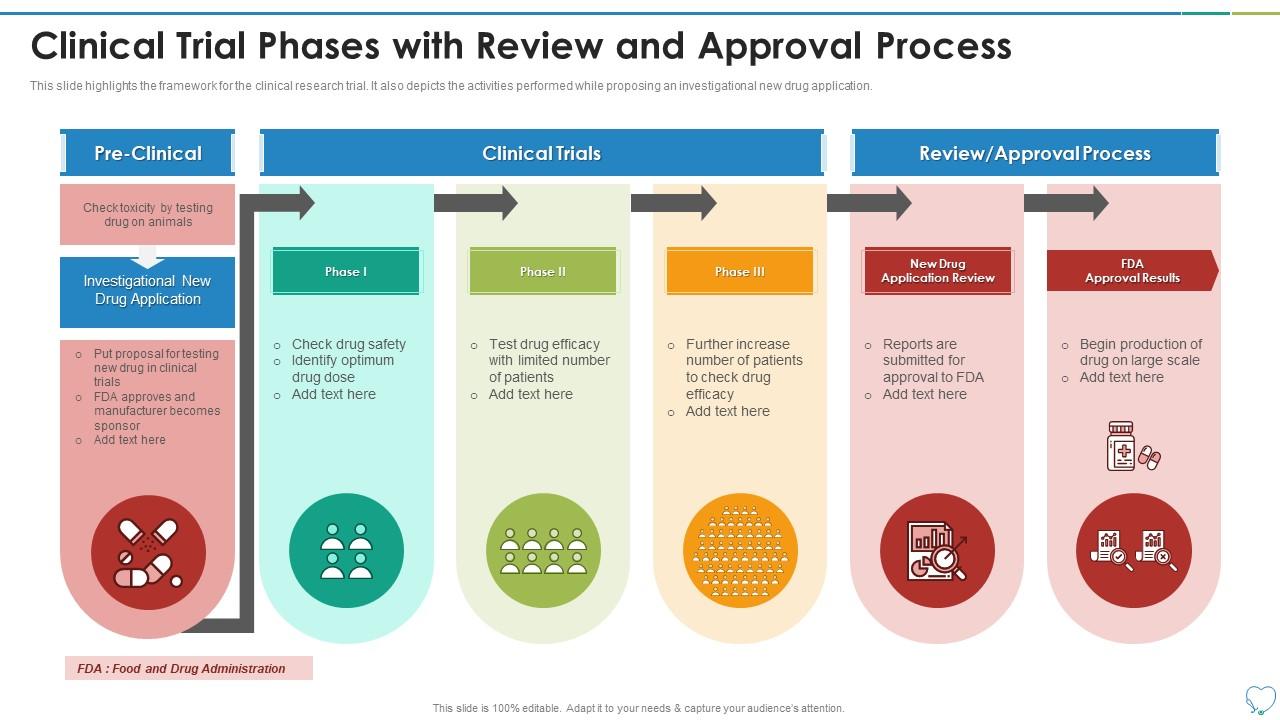
Clinical Trial Phases Review And Approval Process Presentation This narrative review is based on a course in clinical trials developed by one of the authors (djm), and is supplemented by a pubmed search predating january 2011 using the keywords “randomized controlled trial,” “patient clinical research,” “ethics,” “phase iv,” “data and safety monitoring board,” and “surrogate endpoint.”. Phase 0 of a clinical trial is done with a very small number of people, usually fewer than 15. investigators use a very small dose of medication to make sure it isn’t harmful to humans before.

Comments are closed.