Clinical Trial Phases 1 2 3 4 Find Fda Clinical Trial Phases
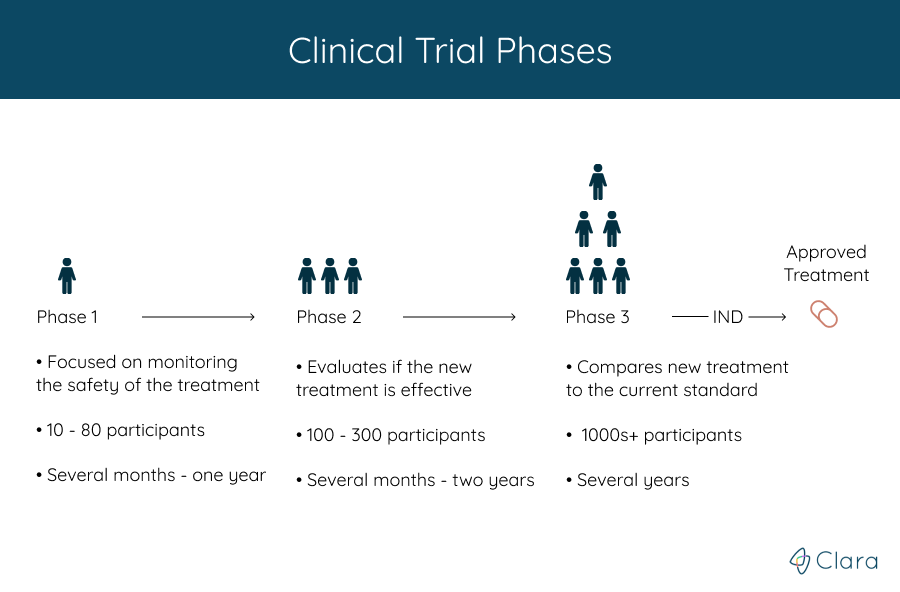
Clinical Trial Phases 1 2 3 4 Find Fda Clinical Trial Phases Clinical trials follow a typical series from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies. what are the clinical trial phases? watch this video to learn about. When phase 3 clinical trials (or sometimes phase 2 trials) show a new drug is more effective and safer than the current standard treatment, a new drug application (nda) is submitted to the food and drug administration (fda) for approval. the nda, which includes data from all the pre clinical and clinical studies, is reviewed by the fda.
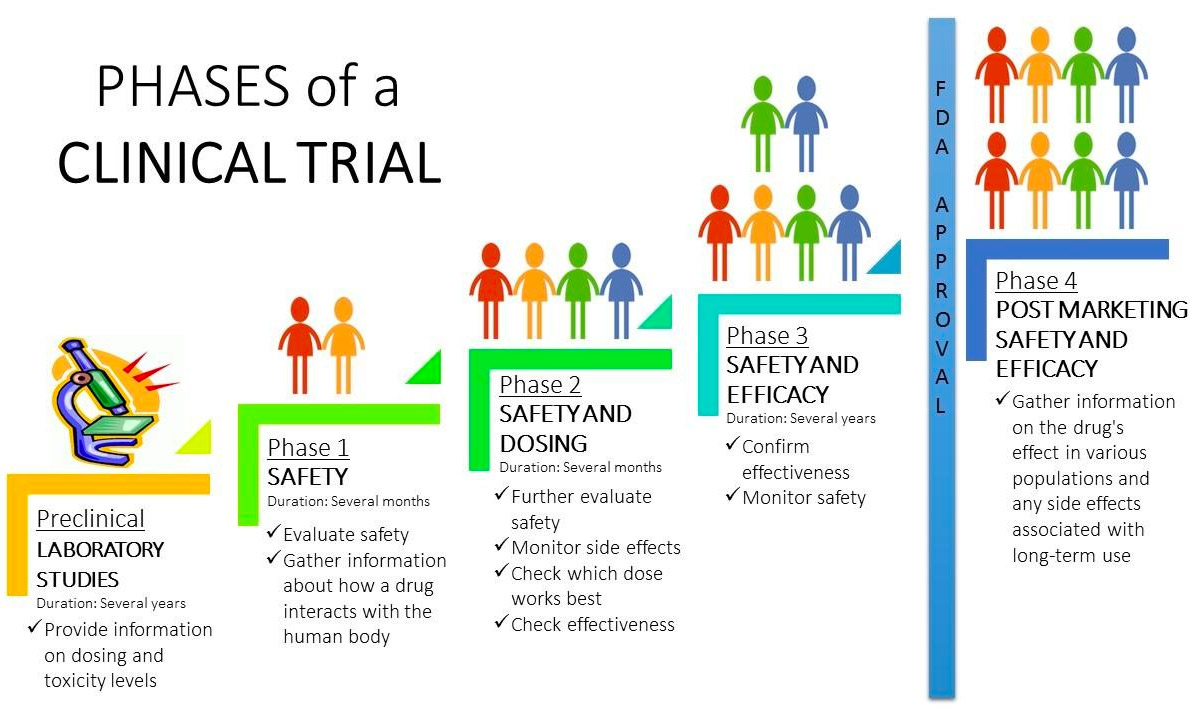
Clinical Trial Phases Diagram If a new treatment is found to be safe in phase i clinical trials, a phase ii clinical trial is done to see if it works in certain types of cancer. the benefit the doctors look for depends on the goal of the treatment. it may mean the cancer shrinks or disappears. Learn about clinical trials for people with cancer. aids clinical trials and information services (actis) or call 1–800–trials–a (1–800–874–2572). locate clinical trials for people. Phase iii of a clinical trial usually involves up to 3,000 participants who have the condition that the new medication is meant to treat. trials in this phase can last for several years. Clinical trials follow a particular timeline, from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies.1 while there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. here we describe the key goals and.

Comments are closed.