Chemical Equation For Magnesium Oxide Magnesium Oxide Lab 2022 10 10

Chemical Equation For Magnesium Oxide Magnesium Oxide Lab 2022 10 10 Empirical formula determination magnesium oxide lab. purpose: to determine the % composition & empirical formula of magnesium oxide. background: we have been talking about the uses of the formulas of compounds as well as how to determine the simplest (empirical) formula of a compound based on chemical analysis. the purpose of this lab is to. The purpose of this experiment is to determine the empirical formula and percent yield of the ionic oxide produced by the reaction of magnesium with oxygen based on experimental data. hypothesis: the empirical formula for magnesium oxide is mgo. percent yield will be 85 100%. introduction: a compound is a chemical combination of two or more.

Chemical Equation For Magnesium Oxide Magnesium Oxide Lab 2022 10 10 Experiment 602: empirical formula . section 1: purpose and summary . determine the empirical formula of magnesium oxide. calculate the mass of oxygen using weighing by difference. calculate the mole of a sample from its mass. in this experiment, students will conduct the reaction between magnesium and oxygen gas. A) the “magnesium oxide” produced in the crucible is not pure white—it is grey 7. the typical procedure generally notes that the formation of the grey powdery substance signals that the reaction is complete and directs students to cool and weigh the crucible. b) magnesium reacts with carbon dioxide to produce carbon and magnesium oxide 8:. The empirical formula of the compound formed is the ratio between the number of moles of elements in the compound. this can be illustrated by the following example (example 1): chemistry 121, spscc (m. dunn, j. chen, t. knowles 2019, 2021, 2022, 2023) in today's lab, you will rapidly react magnesium with oxygen gas under intense heat, then use. 1 mol mg. 24.31 g mg. ( 5b ) mol o = z grams o ×. 1 mol o. 16.00 g o. , where w is the grams of mg used and z is the grams of o incorporated.the empirical formula of magnesium oxide, mgxoy, is written as the lowest whole number ratio between the moles of mg used and moles of o consumed.

Chemical Equation For Magnesium Oxide The empirical formula of the compound formed is the ratio between the number of moles of elements in the compound. this can be illustrated by the following example (example 1): chemistry 121, spscc (m. dunn, j. chen, t. knowles 2019, 2021, 2022, 2023) in today's lab, you will rapidly react magnesium with oxygen gas under intense heat, then use. 1 mol mg. 24.31 g mg. ( 5b ) mol o = z grams o ×. 1 mol o. 16.00 g o. , where w is the grams of mg used and z is the grams of o incorporated.the empirical formula of magnesium oxide, mgxoy, is written as the lowest whole number ratio between the moles of mg used and moles of o consumed. Magnesium oxide (mg o), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). it has an empirical formula of mgo and consists of a lattice of mg 2 ions and o 2− ions held together by ionic bonding. magnesium hydroxide forms in the presence of water (mgo h 2 o →. 7) the reaction between magnesium and oxygen that forms magnesium oxide can be written as the following word equation. in the space below the word equation, write the chemical equation for this reaction and then balance it! (note: the formula for oxygen is o 2). also, indicate the type of reaction. magnesium oxygen magnesium oxide balanced.
Magnesium Oxide Formula What Is The Formula For Magnesium Oxide Magnesium oxide (mg o), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). it has an empirical formula of mgo and consists of a lattice of mg 2 ions and o 2− ions held together by ionic bonding. magnesium hydroxide forms in the presence of water (mgo h 2 o →. 7) the reaction between magnesium and oxygen that forms magnesium oxide can be written as the following word equation. in the space below the word equation, write the chemical equation for this reaction and then balance it! (note: the formula for oxygen is o 2). also, indicate the type of reaction. magnesium oxygen magnesium oxide balanced.
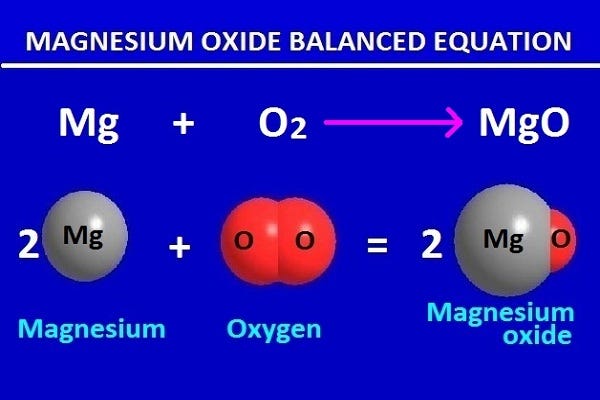
Magnesium Oxide Balanced Equation In Chemistry For Class 9 By Kakali

Comments are closed.