Ccielts Cu Ccwriting Task 1 Cu Table Lesson 2 How To Ccwrite Cu A Ccband 9 Cu O O Uo O O

Complete Ielts 2 Unit 1 Writing Task 1 Therefore we have (still incorrect) 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9 4s 2. correct electron configuration for copper (cu) half filled and fully filled subshell have got extra stability. therefore, one of the 4s2 electrons jumps to the 3d9. this give us the (correct) configuration of: 1s2 2s2 2p6 3s2 3p6 3d10 4s1. The atomic mass of copper is 63.546 u and its density is 8.96 g cm 3. copper is a ductile metal that can be drawn into thin wires. the melting point of copper is 1084.6 °c and its boiling point is 2562 °c. copper has naturally occurring isotopes as well as it has synthetic isotopes too.
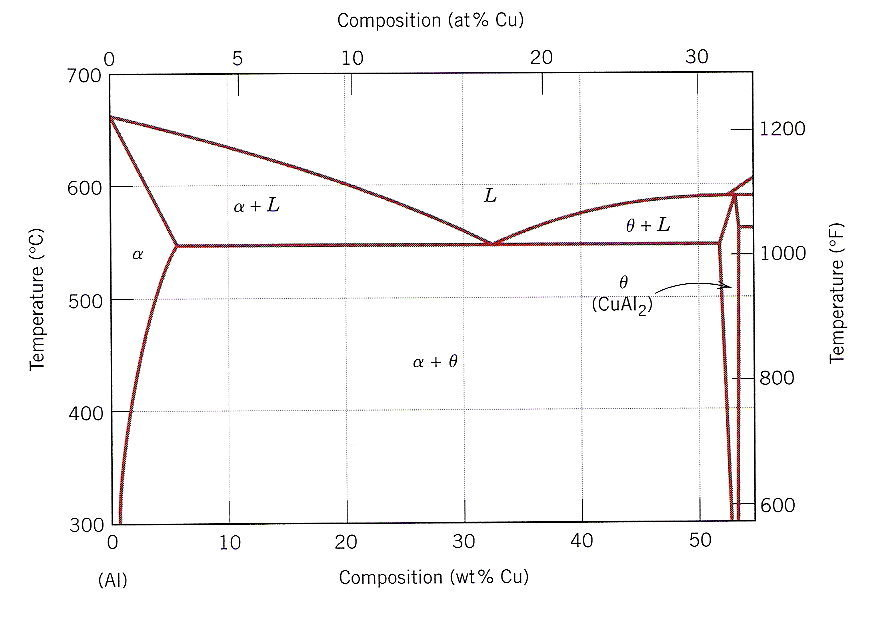
3 Shown Below Is A Portion Of The Al Cu Phase Chegg Interactive periodic table showing names, electrons, and oxidation states. 1 h hydrogen 1.008; 2 he helium 4.0026; 2. 29 cu copper 63.546; 30 zn zinc 65.38;. Atomic number, atomic weight and charge of copper ion. cu – 2e – → cu 2 . the electron configuration of copper ions (cu 2 ) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. copper atoms exhibit 1 and 2 oxidation states. the oxidation state of the element changes depending on the bond formation. Computing molar mass step by step. first, compute the number of each atom in cu: cu: 1. then, lookup atomic weights for each element in periodic table: cu: 63.546. now, compute the sum of products of number of atoms to the atomic weight: molar mass (cu) = ∑ count i * weight i =. count (cu) * weight (cu) =. 1 * 63.546 =. Copper is a chemical element with symbol cu (from latin:cuprum) and atomic number 29. it is a soft, malleable and ductile metal with very high thermal and electrical conductivity. a freshly exposed surface of pure copper has a reddish orange color. it belongs to group 11 of the periodic table having trivial name coinage metals.

How Many Cubic Inches Is 4 Liters Computing molar mass step by step. first, compute the number of each atom in cu: cu: 1. then, lookup atomic weights for each element in periodic table: cu: 63.546. now, compute the sum of products of number of atoms to the atomic weight: molar mass (cu) = ∑ count i * weight i =. count (cu) * weight (cu) =. 1 * 63.546 =. Copper is a chemical element with symbol cu (from latin:cuprum) and atomic number 29. it is a soft, malleable and ductile metal with very high thermal and electrical conductivity. a freshly exposed surface of pure copper has a reddish orange color. it belongs to group 11 of the periodic table having trivial name coinage metals. The writing task 1 table sample has all the elements to get a high score for ielts. there is a to the graph (paraphrasing, not copying, the rubric) and then an that selects key changes trends in the table. it is and thus clear to follow and read, with the paragraphs arranged around age groups (there is usually various ways to organise your. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. in the periodic table, the elements are listed in order of increasing atomic number z. electron configuration of copper is [ar] 3d10 4s1. possible oxidation states are 1,2.

Periodic Table Element Copper Icon Royalty Free Vecto Vrogue Co The writing task 1 table sample has all the elements to get a high score for ielts. there is a to the graph (paraphrasing, not copying, the rubric) and then an that selects key changes trends in the table. it is and thus clear to follow and read, with the paragraphs arranged around age groups (there is usually various ways to organise your. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. in the periodic table, the elements are listed in order of increasing atomic number z. electron configuration of copper is [ar] 3d10 4s1. possible oxidation states are 1,2.

Comments are closed.