Calculate The Emf Of The Cell Zn Zn2 0 1 M Cd2 0 01 M Cd At
31 Find The Emf Of The Cell Zn S Zn 2 0 01m Kcl Saturated Zn 2 1 Click here:point up 2:to get an answer to your question :writing hand:calculate the emf of the cell zn zn2 0001 m cu2 01 m. The emf of a cell : z n 2 h → z n 2 (0.1 m) h 2 (1 a t m) is 0.28 v at 25 ∘ c. calculate ph of the solution at the hydrogen gas electrode. calculate ph of the solution at the hydrogen gas electrode.
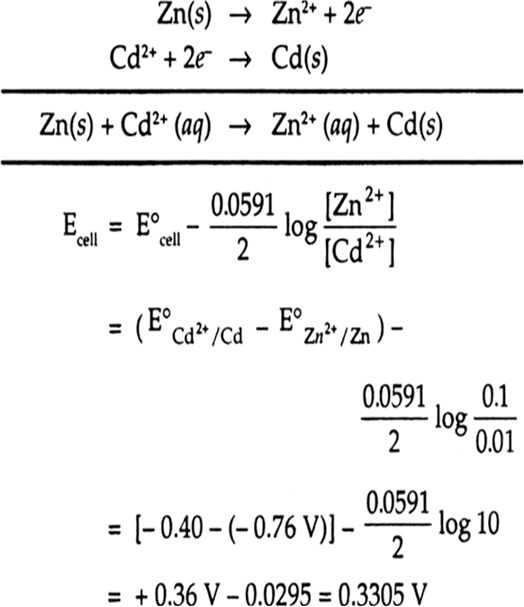
Calculate The Emf Of The Cell Zn Zn2 0 1 M Cd2 0о The emf of the cell, z n | z n 2 (0.01 m) | | f e 2 (0.001 m) | f e at 298 k is 0.2905 v then the value of equilibrium constant for the cell reaction is: view solution. The following curve is obtained when molar conductivity λ m (y axis) is plotted against the square root of concentration c 1 2 (x axis) for two electrolytes a and b. (a) what can you about the nature of the two electrolytes a and b. The emf of the cell with the cell reaction given below is 0.28 v at 25 o c. z n ( s ) 2 h ( a q ) → z n 2 ( a q , 0.1 m ) h 2 ( g , 1 a t m calculate the p h of the hydrogen electrode. Calculate the emf of the following cell at 25 °c: zn | zn2 (0.001 m) || h (0.01 m) | h2(g) (1 bar) | pt(s)watch the full video at: numerade.
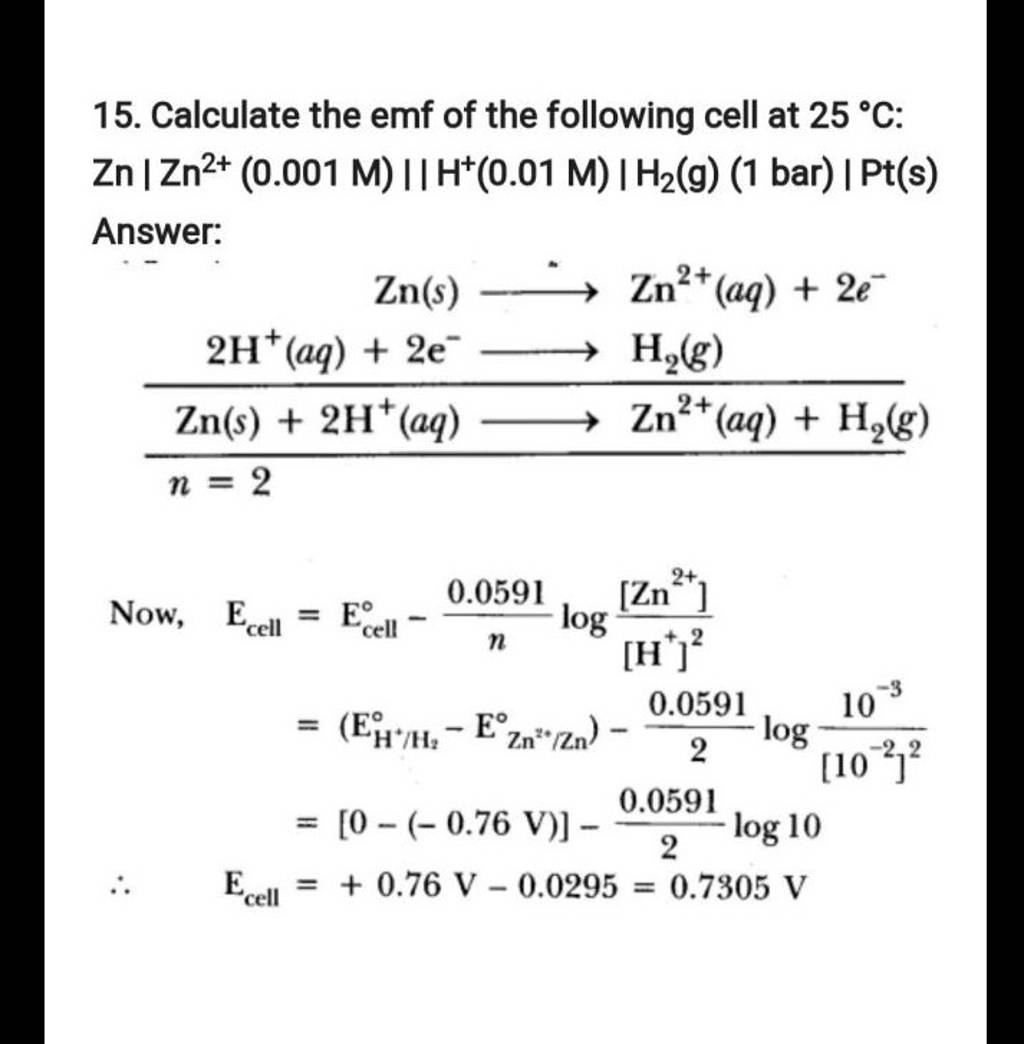
15 юааcalculateюаб юааthe Emfюаб Of The Following юааcellюаб At 25тишc юааznюабтигтиг юааzn2юаб юаа0 The emf of the cell with the cell reaction given below is 0.28 v at 25 o c. z n ( s ) 2 h ( a q ) → z n 2 ( a q , 0.1 m ) h 2 ( g , 1 a t m calculate the p h of the hydrogen electrode. Calculate the emf of the following cell at 25 °c: zn | zn2 (0.001 m) || h (0.01 m) | h2(g) (1 bar) | pt(s)watch the full video at: numerade. A) calculate the emf of the cell represented below: zn zn2 (c = 0.1m) | cu 2 (c = 1m) cu at 25°c given : eºcu = 0.34 v and en = 0.76 v open in app solution. A) calculate the emf of the following cell zn zn2 (0.1m) ii ag (0.2m) ag; given e°zn2 zn = 0.76 v and eºagt ag = 0.8 v 2 b) a cu rod is placed in 0.01 m cuso4 solution at 298k. calculate the potential of the electrode assuming that salt is dissociated to 90 % at this dilution. given e° for cu2 cu = 0.34 v.

Solved A Calculate The Emf Of The Following Cell Zn Zn2 Chegg A) calculate the emf of the cell represented below: zn zn2 (c = 0.1m) | cu 2 (c = 1m) cu at 25°c given : eºcu = 0.34 v and en = 0.76 v open in app solution. A) calculate the emf of the following cell zn zn2 (0.1m) ii ag (0.2m) ag; given e°zn2 zn = 0.76 v and eºagt ag = 0.8 v 2 b) a cu rod is placed in 0.01 m cuso4 solution at 298k. calculate the potential of the electrode assuming that salt is dissociated to 90 % at this dilution. given e° for cu2 cu = 0.34 v.

Ag Calculate The Emf Of The Cell Zn Zn2 0 008m Cr3 0 01m I

Comments are closed.