Biochemistry Lecture 8 Protein Folding And Energetics Flashcards

Biochemistry Lecture 8 Protein Folding And Energetics Flashcards Study with quizlet and memorize flashcards containing terms like secondary structures go to form a hydrophobic core in tertiary structures, thermodynamics of protein folding!, factors that influence protein structures and more. 1) parallel n and c terminals are parallel, (not the c=o h n hydrogen bonds). 2) antiparallel c and n terminals are opposite between columns (hydrogen bonds parallel ironically). antiparallel can be linked from c to n across columns, while parallel requires an extended sequence to return back to the top.
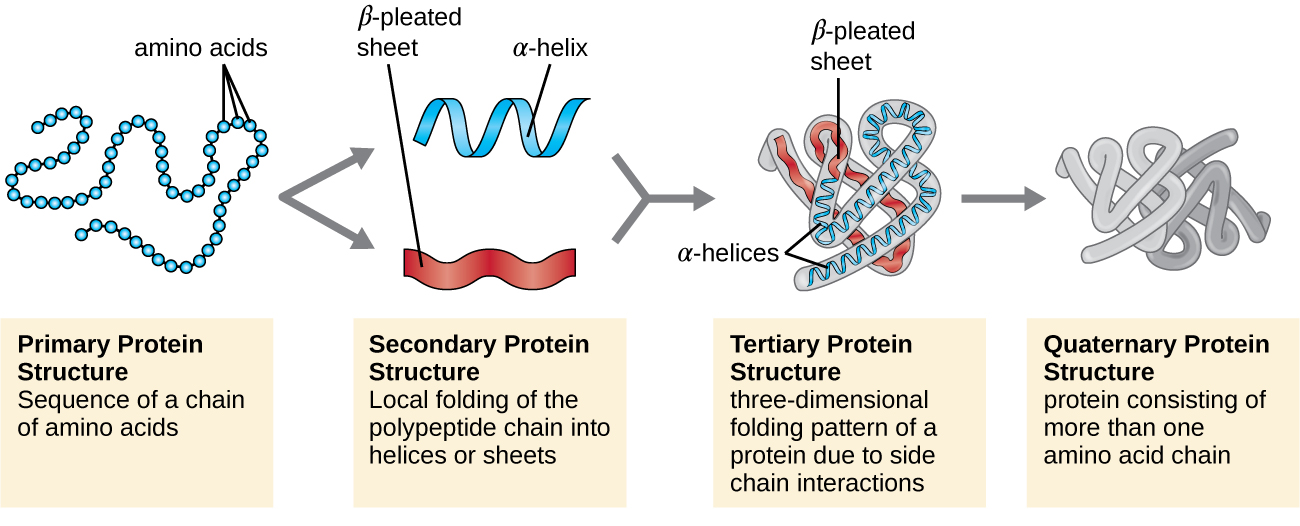
Protein Structure Biochemistry At Charles Wilson Blog 1,2,4,5,7,8,9 lecture 20 (10 28 20) protein structure a. stability 1. two state model 2. energetics 3. denaturation 4. methods to study b. protein folding 1.evidence anfinsen 2.protein folding pathways 3.mechanism; in vitro vs. in vivo a. kinetics b. thermodyna mics 4.diseases c. prediction d. dynamics what are these observables? unfolding. Prions are composed of. protein only. rna surrounded by a lipid membrane and protein coat. protein and dna. protein and rna. protein only. the correctly folded three dimensional configuration of a protein is determined primarily by the. primary sequence of its amino acids. which of the following groups anchors proteins to the outer surface. Study protein folding and misfolding using smart web & mobile flashcards created by top students, teachers, and professors. lecture 3: proteins and ph, lecture 4. Lecture 8: protein folding 1. freely sharing knowledge with learners and educators around the world. learn more. mit opencourseware is a web based publication of virtually all mit course content. ocw is open and available to the world and is a permanent mit activity.

Lecture 8 Protein Folding Flashcards Quizlet Study protein folding and misfolding using smart web & mobile flashcards created by top students, teachers, and professors. lecture 3: proteins and ph, lecture 4. Lecture 8: protein folding 1. freely sharing knowledge with learners and educators around the world. learn more. mit opencourseware is a web based publication of virtually all mit course content. ocw is open and available to the world and is a permanent mit activity. Study these flashcards. a. ptms can alter interactions between amino acids, changing the tertiary or quaternary structure. study lecture 5 protein structure and function flashcards from devina ruthland's class online, or in brainscape's iphone or android app. learn faster with spaced repetition. Bio1332 biochemistry lecture eight protein folding and stability. understand the physical basis of the various forces that hold proteins together. understand that protein structure is determined by primary sequence. understand that the folded native conformation is the most energetically favourable. appreciate that protein folding is a.

Comments are closed.