Ap Ch 19 Entropy And Gibbs Free Energy

Gibbs Free Energy Entropy Enthalpy Equilibrium Constant K Youtube Entropy. measure of disorder or randomness in system. second law of thermodynamics. states that spontaneous processes always proceed in such way that entropy of universe increases. free energy. energy available to do work. Δg = Δh tΔs. gibbs free energy equation. spontaneous reaction. Study with quizlet and memorize flashcards containing terms like gibbs free energy, enthalpy, entropy and more. ch 19. 30 terms. chapter 14: entropy and free.

Gibbs Free Energy Definition Equation Unit And Example This video provides a basic introduction into gibbs free energy, entropy, and enthalpy. it explains how to calculate the equilibrium constant k given the st. Gch18 19 gibbs free energy (g) another thermodynamic function that helps determine whether a reaction is spontaneous is gibbs free energy, also known as free energy. two driving forces in the nature (one related to the energy change, and another related to the dissorder change) are combined in one equation. Gibbs free energy equation at a constant temperature. g= h t s. in most situations, to get s to the same unit as h (kj), what must you do to s? divide by 1000. to know the amount of free energy in the system, which formula do you use? g= h t s. if h<t s, is it entropy or enthalpy driven? entropy driven. if h>t s, is it entropy or enthalpy driven?. Entropy is a thermodynamic function that describes the number of arrangements (positions and or energy levels) that are available to a system existing in a given state. entropy is closely related to probability. the key concept is that the more ways a particular state can be achieved; the greater is the likelihood (probability) of finding that.
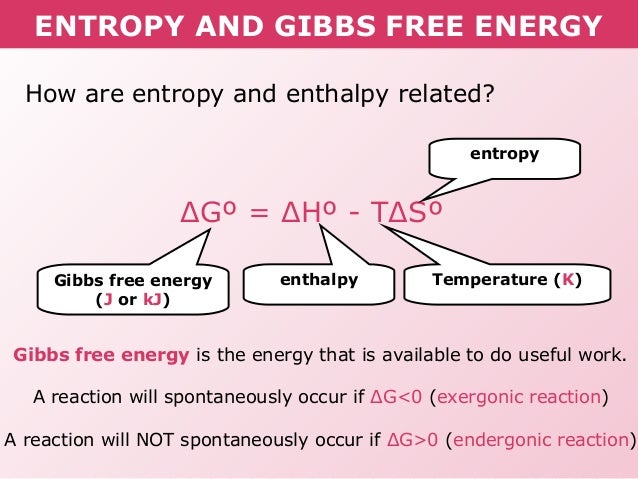
How Is Gibbs Free Energy Related To Enthalpy And Entropy Socratic Gibbs free energy equation at a constant temperature. g= h t s. in most situations, to get s to the same unit as h (kj), what must you do to s? divide by 1000. to know the amount of free energy in the system, which formula do you use? g= h t s. if h<t s, is it entropy or enthalpy driven? entropy driven. if h>t s, is it entropy or enthalpy driven?. Entropy is a thermodynamic function that describes the number of arrangements (positions and or energy levels) that are available to a system existing in a given state. entropy is closely related to probability. the key concept is that the more ways a particular state can be achieved; the greater is the likelihood (probability) of finding that. This video focuses on the concepts of entropy, entropy calculations, gibbs free energy, calculation of delta g, thermodynamic favorability, and kinetic contr. Determine whether each reaction is spontaneous or not by calculating the Δsuniv based on the values of Δ h ° rxn, Δ s ° rxn, and t. assume that all the components in the reaction are in their standard states. a) Δ h ° rxn = 126 kj; Δ s ° rxn = 231 j k; t = 298 k. b) Δ h ° rxn = 5 kj; Δ s ° rxn = 139 j k; t = 298 k.

Comments are closed.