Ag Agcl Reference Electrode Drawing

Schematic Cross Section Of A Simple Ag Agcl Reference Electrode Another common ag agcl electrode uses a solution of 3.5 m kcl and has a potential of 0.205 v at 25 o c. as you might expect, the potential of a ag agcl electrode using a saturated solution of kcl is more sensitive to a change in temperature than an electrode that uses an unsaturated solution of kcl. a typical ag agcl electrode is shown in. 2. silver silver chloride (ag agcl) the silver silver chloride reference electrode is composed of a silver wire, sometimes coated with a layer of solid silver chloride, immersed in a solution that is saturated with potassium chloride and silver chloride. the pertinent half reaction is. agcl(s) e − ⇔ ag(s) cl − (sat’d) with a value.
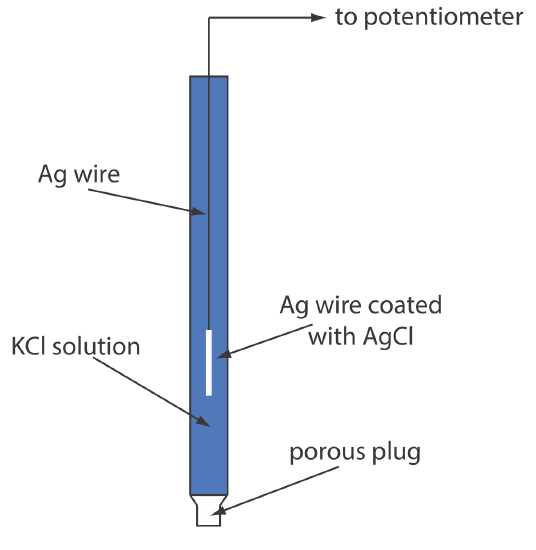
23 1 Reference Electrodes Chemistry Libretexts The ag agcl reference electrode is a popular and widely used electrode in electrochemical measurements. its structure is simple, yet effective, consisting of a silver wire coated with a layer of silver chloride. the electrode works based on the redox reaction between ag and agcl, which acts as a reversible redox couple. Section 4. reference electrodes. 26. if the two electrodes are of the same type (e.g., ag agcl vs. ag agcl, or calomel vs. calomel) the meter should read 0 ±20 mv. if your reading for any pair of electrodes is significantly different, you should have another electrode of the same type handy to help distinguish which of the two is bad. Simple video illustrating the relatively simple construction of an ag agcl reference electrode. \[ag(s) cl^–(aq) →agcl(s) e^–\] this electrode usually takes the form of a piece of silver wire coated with agcl. the coating is done by making the silver the anode in an electrolytic cell containing hcl; the ag ions combine with cl – ions as fast as they are formed at the silver surface. the other common reference electrode is.

Comments are closed.