A New Look At Protein Misfolding

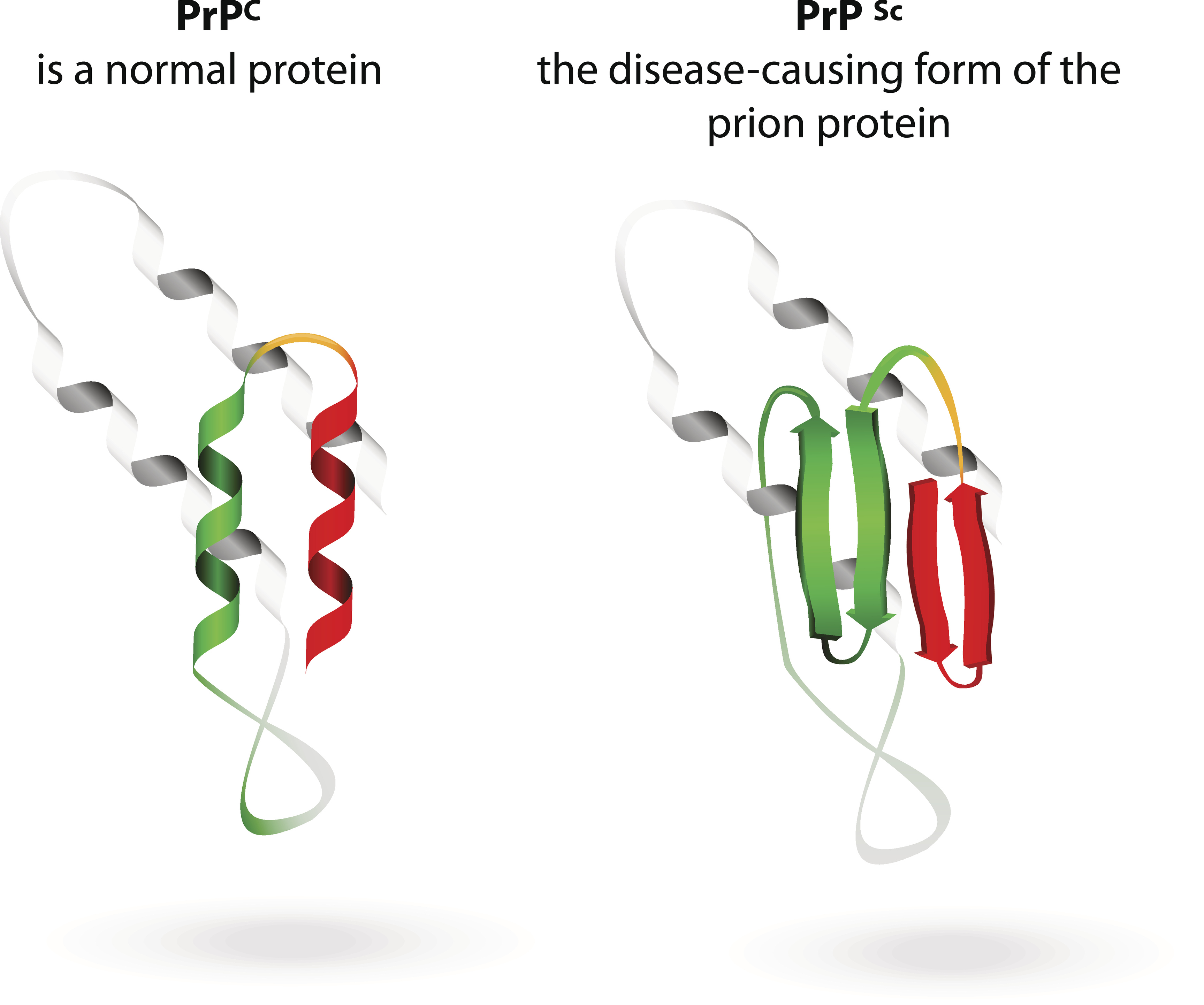
A New Look At Protein Misfolding A new look at protein misfolding protein chemistry: fluorescence method reveals details of disease related process by celia henry arnaud june 6, 2011 | a version of this story appeared in volume. Researchers have used an established fluorescence technique to study misfolded structures that arise in multidomain proteins (nature, doi: 10.1038 nature10099).most studies of protein misfolding have focused on single domain proteins, but the majority of eukaryotic proteins are multidomain.
Protein Misfolding That Propagates And The Mechanisms Of Protein misfolding also causes proteins to stray away from their original native folding trajectory, illustrated by the presence of a side of the energy funnel where proteins become trapped in. Telegram. a groundbreaking study at umass amherst has decoded how sugars attached to proteins guide their correct folding, shedding light on potential treatments for diseases caused by protein misfolding. this protein (red) has been glycosolated with glycans (blue and green). credit: umass amherst. team’s approach reveals crucial role played. Huang and colleagues now identify daxx as a member of a new class of protein folders that behave unlike traditional chaperones (huang et al., 2021). daxx plays roles in many processes including p53 tumor suppressor regulation, nucleosome deposition, and apoptotic signaling (mahmud and liao, 2019). like 44 other human proteins, daxx possesses an. In a new study published april 20 in nature cell biology, researchers at stanford university discovered a previously unknown cellular pathway for clearing misfolded proteins from the nucleus, the.

A Closer Look At The Root Of Protein Misfolding Technology Networks Huang and colleagues now identify daxx as a member of a new class of protein folders that behave unlike traditional chaperones (huang et al., 2021). daxx plays roles in many processes including p53 tumor suppressor regulation, nucleosome deposition, and apoptotic signaling (mahmud and liao, 2019). like 44 other human proteins, daxx possesses an. In a new study published april 20 in nature cell biology, researchers at stanford university discovered a previously unknown cellular pathway for clearing misfolded proteins from the nucleus, the. Once internalized, misfolded protein aggregates can act as seeds to propagate protein misfolding in the new host cell. the cellular spreading and propagation of protein misfolding have been already reported for various proteins, such as α syn, tau, htt and prp [41,42,60]. in the case of proteins sitting on the cell surface, such as prp, cell. The loss of function and toxicity that is associated with the accumulation of mutant protein in the er leads to genetic and developmental disorders and neurodegenerative diseases. environmental or.

Comments are closed.