11 Which Of The Following Would Precipitate The Ca2 And Mg2 Found In Hard Water A 82 B Po4
Solved A Sample Of Ground Water Has 150 Mg L Of Ca2 And 60 Mg L Of 11. which of the following would precipitate the ca2 and mg2 found in hard water? a. 82 b. po4 c. so4^2 d. ch3coo 12. which of the following will be mo. Our expert help has broken down your problem into an easy to learn solution you can count on. question: 2) which of the following would precipitate the ca2 and mg2 found in hard water? a) s2 b) pos c) so, d) ch,coo. here’s the best way to solve it. answer is (b) po43 (phosphate) phosphate componds of calcium and magnes ….
/precipitate-589cb8953df78c47581a9014.jpg)
Precipitate Definition And Example In Chemistry The main ions contributing to water hardness include ca2 , mg2 , and fe3 . their presence makes it difficult for soaps to lather and causes a 'scum' to form. the main problem with water hardness in industrial water systems is that the buildup of solid caco3 which precipitates out and causes thick deposits to form in pipes or other appliances. Study with quizlet and memorize flashcards containing terms like hard water, which contains mg2 and ca2 ions, tends to form a ring in a bathtub due to its reaction with the soluble anions in soap. the formation of this insoluble material is an example of select one: a. a precipitation reaction. b. a redox reaction. c. a decomposition reaction. d. an acid base reaction. e. a. Problem sets built by lead tutors expert video explanations. hard water often contains dissolved ca2 and mg2 ions. one way to soften water is to add phosphates. the phosphate ion forms insoluble precipitates with calcium and magnesium ions, removing them from solution. a solution is 0.050 m in calcium chloride and 0.085 m in magnesium nitrate. Tests for cations. 25 terms. joshroycheesy. 1 5. study with quizlet and memorize flashcards containing terms like hard water is water that contains specific ions by passing certain areas with specific rocks, for example, limestone, chalk and gypsum, ca2 and mg2 ions, mix it with soap and more.
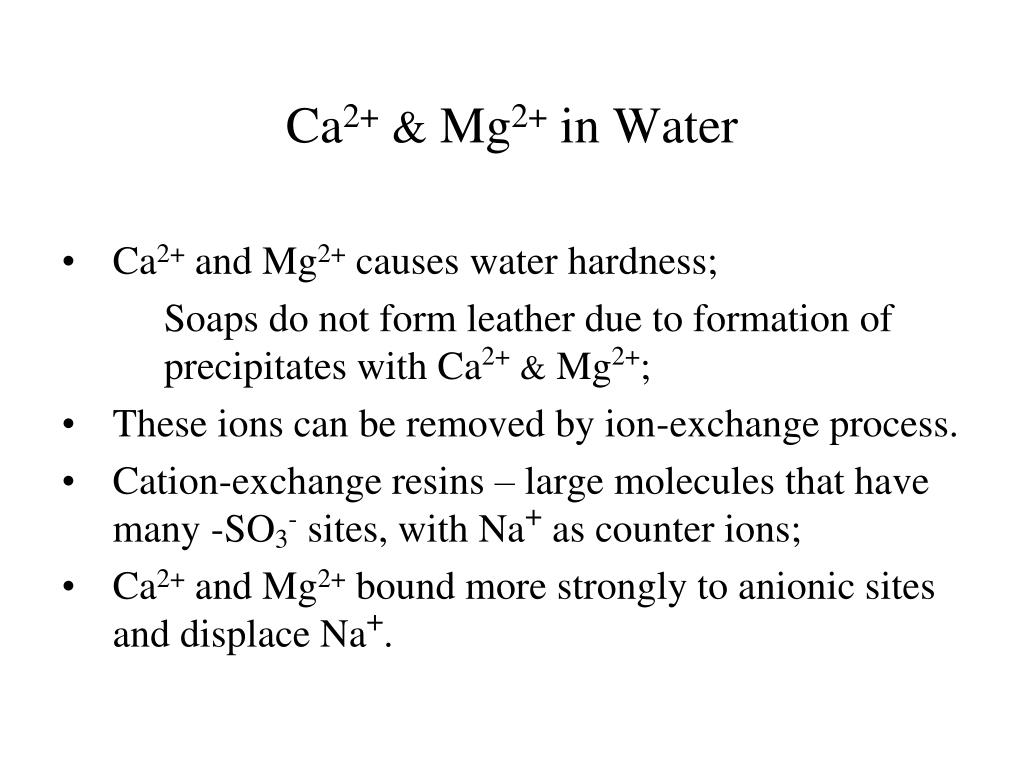
Ppt The Representative Elements Groups 1a 4a Powerpoint Problem sets built by lead tutors expert video explanations. hard water often contains dissolved ca2 and mg2 ions. one way to soften water is to add phosphates. the phosphate ion forms insoluble precipitates with calcium and magnesium ions, removing them from solution. a solution is 0.050 m in calcium chloride and 0.085 m in magnesium nitrate. Tests for cations. 25 terms. joshroycheesy. 1 5. study with quizlet and memorize flashcards containing terms like hard water is water that contains specific ions by passing certain areas with specific rocks, for example, limestone, chalk and gypsum, ca2 and mg2 ions, mix it with soap and more. Hard water is water containing high amounts of mineral ions. the most common ions found in hard water are the metal cations calcium (ca 2 ) and magnesium (mg 2 ), though iron, aluminum, and manganese may also be found in certain areas. these metals are water soluble, meaning they will dissolve in water. Video answer: hi everyone. according to the question we have to find the compound that precipitated ce to present institute. in hard work options. given up. yes two minus p. 0. 4 3 . yes. so food to minus and the h three c. o. mania. we're only four.

Solved Which Of The Following Could Be Used To Precipitate Both Mg2 Hard water is water containing high amounts of mineral ions. the most common ions found in hard water are the metal cations calcium (ca 2 ) and magnesium (mg 2 ), though iron, aluminum, and manganese may also be found in certain areas. these metals are water soluble, meaning they will dissolve in water. Video answer: hi everyone. according to the question we have to find the compound that precipitated ce to present institute. in hard work options. given up. yes two minus p. 0. 4 3 . yes. so food to minus and the h three c. o. mania. we're only four.

Solved Text Resources Hint Check A The Total Concentration Of Ca2

Comments are closed.