10 5 Molecular Orbital Theory Chemistry Libretexts
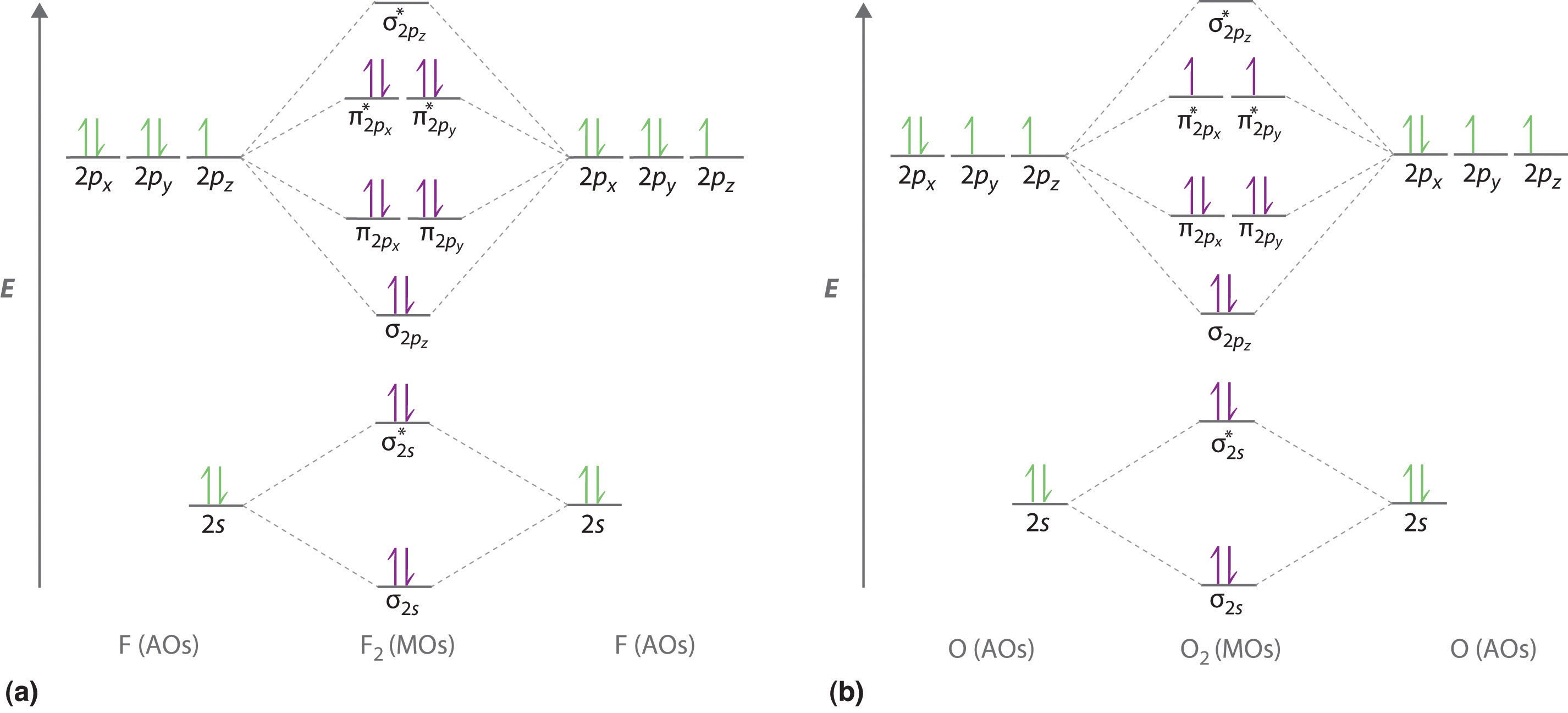
10 5 Molecular Orbital Theory Chemistry Libretexts Molecular orbital theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals (s, p, d …) and hybrid orbitals (sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds. Use molecular orbital theory to predict molecular geometry for simple triatomic systems. rationalize molecular structure for several specific systems in terms of orbital overlap and bonding. understand the origin of aromaticity and anti aromaticity in molecules with π bonding. valence bond (vb) theory gave us a qualitative picture of chemical.
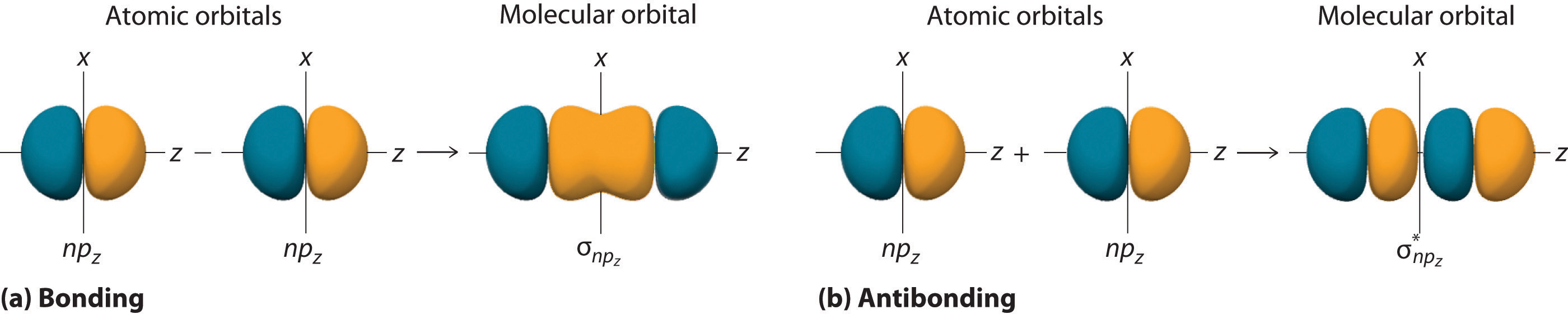
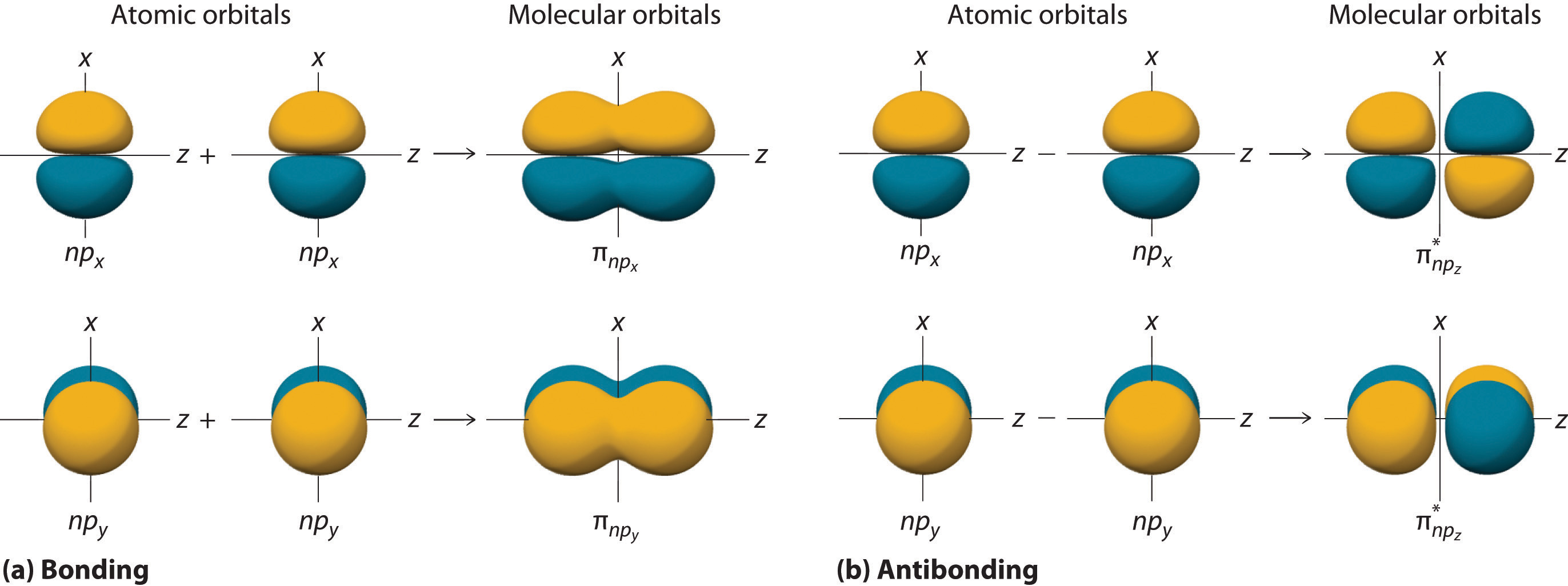
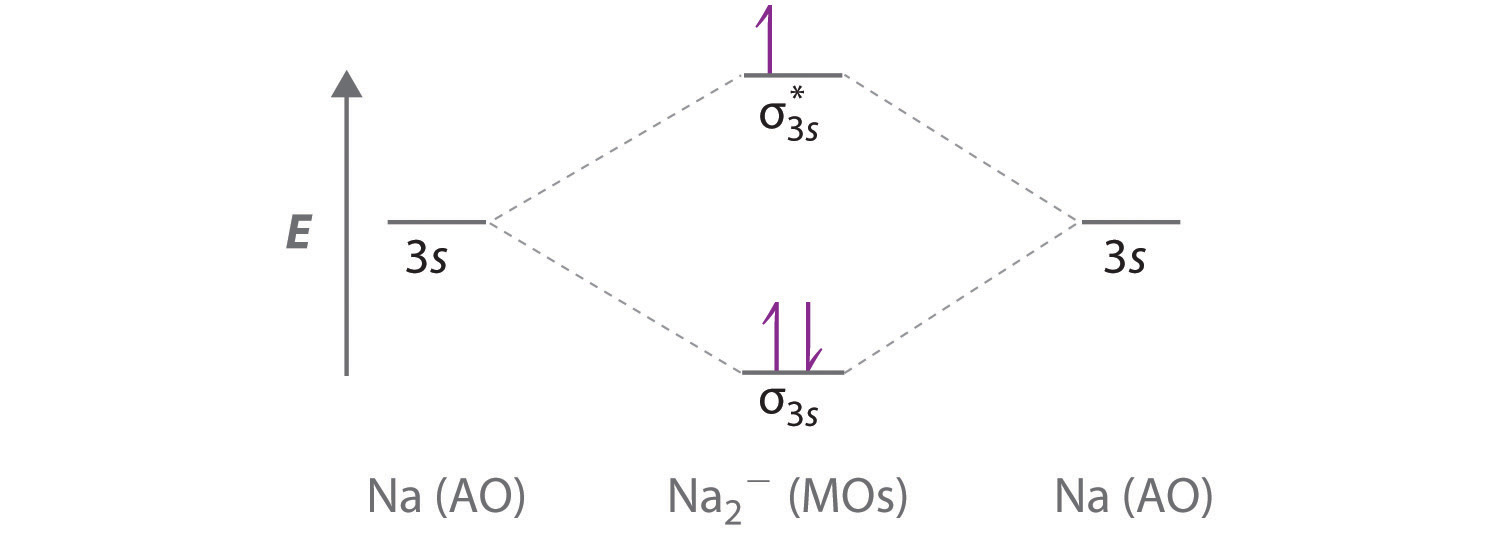
10 5 Molecular Orbital Theory Chemistry Libretexts Figure 5.5.9 5.5. 9: the molecular orbital energy diagram predicts that h 2 will be a stable molecule with lower energy than the separated atoms. a dihydrogen molecule contains two bonding electrons and no antibonding electrons so we have. bond order in h2 = (2 − 0) 2 = 1 bond order in h 2 = (2 − 0) 2 = 1. The side by side overlap of two p orbitals gives rise to a pi (π) bonding molecular orbital and a π* antibonding molecular orbital, as shown in figure 5.31. in valence bond theory, we describe π bonds as containing a nodal plane containing the internuclear axis and perpendicular to the lobes of the p orbitals, with electron density on either side of the node. In chemistry, a molecular orbital ( ɒrbədl ) is a mathematical function describing the location and wave like behavior of an electron in a molecule. this function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The four chemically important types of atomic orbital correspond to values of ℓ ℓ = 0, 1, 2, and 3. orbitals with ℓ ℓ = 0 are s orbitals and are spherically symmetrical, with the greatest probability of finding the electron occurring at the nucleus. all orbitals with values of n > 1 and ℓ ℓ = 0 contain one or more nodes.
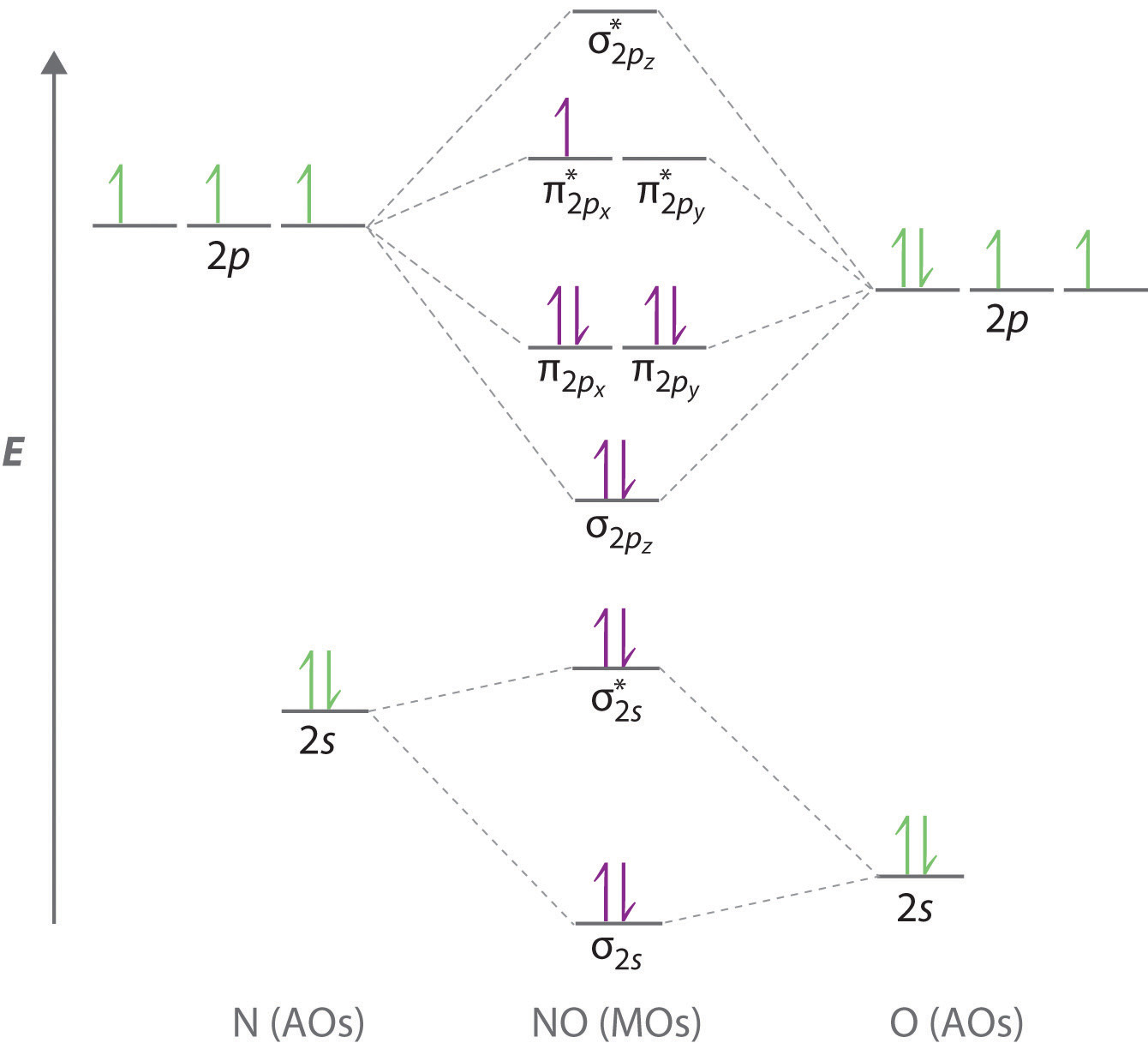
10 5 Molecular Orbital Theory Chemistry Libretexts In chemistry, a molecular orbital ( ɒrbədl ) is a mathematical function describing the location and wave like behavior of an electron in a molecule. this function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The four chemically important types of atomic orbital correspond to values of ℓ ℓ = 0, 1, 2, and 3. orbitals with ℓ ℓ = 0 are s orbitals and are spherically symmetrical, with the greatest probability of finding the electron occurring at the nucleus. all orbitals with values of n > 1 and ℓ ℓ = 0 contain one or more nodes. The lowest unoccupied molecular orbital of the carbon monoxide molecule is a π antibonding orbital that derives from the 2p orbitals of carbon (left) and oxygen (right) this page titled is shared under a cc by sa 4.0 license and was authored, remixed, and or curated by chemistry 310 (wikibook) via source content that was edited to the style. Molecular orbital theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals (s, p, d …) and hybrid orbitals (sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds.
10 5 Molecular Orbital Theory Chemistry Libretexts The lowest unoccupied molecular orbital of the carbon monoxide molecule is a π antibonding orbital that derives from the 2p orbitals of carbon (left) and oxygen (right) this page titled is shared under a cc by sa 4.0 license and was authored, remixed, and or curated by chemistry 310 (wikibook) via source content that was edited to the style. Molecular orbital theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals (s, p, d …) and hybrid orbitals (sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds.
10 5 Molecular Orbital Theory Chemistry Libretexts

Comments are closed.